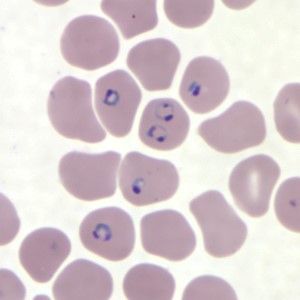
 Figure 1. Human red blood cells infected with P. falciparum. Image source: Centers for Disease Control and Prevention. Figure 1. Human red blood cells infected with P. falciparum. Image source: Centers for Disease Control and Prevention. The host, the vector, and the parasite Malaria is caused by a single-celled eukaryotic (that is, not a virus or a bacteria, but a cellular organism whose nucleus is enclosed in a membrane) parasite in the genus Plasmodium. While five species in this genus are known to cause the disease, P. falciparum is most often responsible. The parasite’s life cycle requires both human and mosquito hosts to complete its life cycle. The Plasmodium is in its motile stage when it first encounters a human, when the parasite is injected along with the mosquito’s salivary secretions into the human’s skin. They travel to the liver, where they enter a liver cell (hepatocyte) and undergo several divisions, producing thousands of cellular descendants. These descendants are then released into the human’s bloodstream where they enter red blood cells (Figure 1). It is at this stage in the parasite’s life cycle, about a week after the original mosquito bite, that the human host is most likely to start experiencing symptoms of malaria, which can include a severe fever, chills, and digestive issues. Some of the parasite cells now within the human’s red blood cells will then mature into male and female forms, which can then be ingested by another mosquito when it bites an infected human. At this point, the male and female forms of the parasite fuse, replicate, and move to the salivary glands of the mosquito, where it can then be injected into a new human host when the mosquito bites. The parasite can also be transmitted from a pregnant human host to their unborn child but is not transmitted from an infected female mosquito to her offspring. Thus preventing mosquito bites is not only key to preventing infections of the primary, human hosts, it’s also critical to stopping the spread from human to human, by way of the mosquito vector.
 Figure 3. Spraying Bti on a stagnant pool of water to kill mosquito larvae. Photo kindly provided by Rob McCann Figure 3. Spraying Bti on a stagnant pool of water to kill mosquito larvae. Photo kindly provided by Rob McCann Between 2016 and 2018, McCann and colleagues[i] conducted a randomized trial covering 65 villages in the Majete region of southern Malawi to test two additional interventions: housing improvements and larval source management. Housing improvements focused on closing gaps through which mosquitoes can enter a house; open eaves were closed with mud and bricks and screens were added to windows (Figure 2). Larval source management involved draining or filling pools of standing water that weren’t needed; pools of water that served some function (such as providing water for livestock) were sprayed with the insecticide Bti (Bacillus thuringiensis serotype israelensis, a bacteria that is known to be toxic to mosquitoes and a few other Dipteran families; Figure 3). Villages were assigned to either intervention, both, or neither, but all villages continued to receive malaria prevention support from the National Malaria Control Program (NMCP) of Malawi, which involved distribution of bed nets and access to treatment. To assess the effects of these interventions, the research team then set up traps for host-seeking mosquitoes inside and outside people’s homes to monitor the mosquito population, sampled for larvae in bodies of water to track the prevalence of larvae, and used rapid malaria diagnostic tests and interviews to track infections in the local population. In the end, the researchers failed to see a statistically significant effect of these interventions. McCann suspects this could be attributed to overall improvements made in the control areas which did not receive either housing improvements of larval source management, but did receive interventions from the NMCP, which relies on bed net distribution as its primary intervention. Importantly, at all stages of this project, the members of the 65 villages that the researchers engaged with were in charge of implementing the interventions in their communities. Community members trained under the title of health animators led the effort to implement these interventions and were trained about the biology of the parasite and the vector, information that they relayed to the rest of their community through workshops held every other week. Since community members were trained to carry out the interventions themselves, even now that the study period is over, many continue to work on improving houses to limit mosquito entry. References
Katie Reding is a PhD student in the Pick lab at the University of Maryland, studying the regulatory interactions underlying segmentation of the milkweed bug embryo. [i] Importantly, this project was carried out by a vast network of collaborators, including the College of Medicine at the University of Malawi in Blantyre, Malawi; the Academic Medical Centre at the University of Amsterdam in Amsterdam, The Netherlands; the Liverpool School of Tropical Medicine in Liverpool, United Kingdom, Lancaster University in Lancaster, United Kingdom, The Hunger Project-Malawi, African Parks in Majete, Malawi, and the Ministry of Health of Malawi Comments are closed.
|
Categories
All
Archives
March 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290



 RSS Feed
RSS Feed




