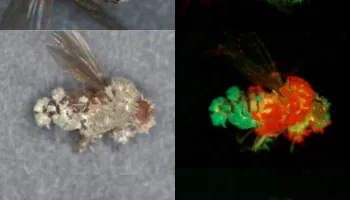
Genetics, Genomics, Developmental Biology & Medical Entomology
Much of the early work in fields like genetics, neurobiology, endocrinology, gene expression, sex determination, and translational control was conducted using insects. Today, insects continue to serve as important models for developmental biology, physiology, neural and molecular correlates of behavior, vector biology, and evolutionary biology.

The completion of the Drosophila genome and other insect genetic tools has maintained the place of insects at the forefront of research on the structure, function, mapping, organization, expression, and evolution of genomes.
Several Department of Entomology faculty members use insects to answer basic scientific questions ranging from the function of individual genes and gene networks to the genome-wide effect of genetically modified crops on agricultural pests. While many of these studies use Drosophila melanogaster and several species of mosquitoes as model systems, othes include Lepidoptera, the Colorado potato beetle and other beetles, brown marmorated stink bug, milkweed beetles.
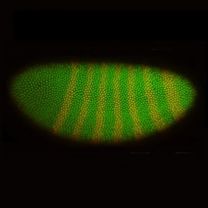 Department faculty members partner with local and state agencies monitor mosquito populations in urban landscapes as well as federal agencies including the National Institutes of Health to conduct tests of previously undiagnosed diseases in the model organism D. melanogaster. This exciting research provides students and postdocs with valuable experience using state of the art genetic and computational tools in the lab as well as field experience ranging from local research farms to the sub-Saharan African country of Burkina Faso.
Department faculty members partner with local and state agencies monitor mosquito populations in urban landscapes as well as federal agencies including the National Institutes of Health to conduct tests of previously undiagnosed diseases in the model organism D. melanogaster. This exciting research provides students and postdocs with valuable experience using state of the art genetic and computational tools in the lab as well as field experience ranging from local research farms to the sub-Saharan African country of Burkina Faso.