|
Alison Post (BS Biology 2014) received the NSF Graduate Research Fellowship. Alison is the former Lab Manager for the Lamp Lab and is now an ecology graduate student at Colorado State University. Check out the news here: https://cmns.umd.edu/news-events/features/3483. Congratulations Alison!
Entomology Graduate Students Nathalie Steinhauer and Meghan McConnell Collect Bees from California Almond Farm for USDA Testing – Los Angeles Times
Check out the full article here: goo.gl/6hSFZp Entomology’s David O’Brochta Comments on the Testing of Genetically Modified Mosquitoes – NBC News
Check out the full article here: goo.gl/SnlvgQ One of our recently graduated students Dr. Dilip Venugopal (now a AAAS fellow) has coauthored a paper related to brown- marmorated Stink Bugs in PLOS ONE. Check it out at goo.gl/CvAZ2c . You can also read his views here: goo.gl/1ZyLCf
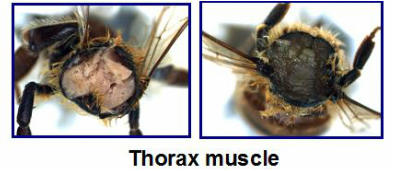 Figure 1. the thorax muscle tissue of a healthy bee (left) compared to that of a "Black Bee" Figure 1. the thorax muscle tissue of a healthy bee (left) compared to that of a "Black Bee" Dr. Humberto Boncristiani is a post-doc in the vanEngelsdorp bee lab at the University of Maryland. He is currently studying a variety of honey bee viruses, with a particular focus on picorna-like viruses. His interest in honey bees came from his father, who keeps bees in Brazil, and his virology background comes from his work on human viruses at the University of Sao Paulo. The inspiration for his transition from humans to honey bees came in 2007 when Diana Cox-Foster published a paper documenting an association between Israeli acute paralysis virus (IAPV) and colony collapse disorder (CCD). IAPV just so happened to belong to the same group of viruses that Humberto was studying in humans. This prompted him to make the jump to studying honey bee viruses. Picornaviruses have a wide range of hosts, from honey bees to humans. They are of particular interest because of the manner in which they replicate. Picornaviruses have an Internal Ribosome Entry Site (IRES) analogous to that of their host’s mRNA. The IRES is a complex part of the viral RNA genome used to confuse the cellular machinery and inciting the ribosome to start translation of the virus proteins. In order to prevent the cell from producing its own proteins, the picornavirus cleaves an important protein from the cellular mRNA making it unrecognizable to the ribosome (Boncristiani et al., 2009). Finally, the picornavirus uses the cell’s ribosomes to replicate itself by the thousands. The virus transcriptase lacks the ability to proofread itself, leading to higher mutational rates in the copies it makes. This results in a cloud of genetically diverse viruses, most of which can cause physical symptoms and infect other cells. The incredible genetic diversity in these viruses makes them dangerous pathogens that hosts have a difficult time evolving resistance to, which is why they have such a profound effect on bee colonies. Some of Humberto’s current work is based on a phenomenon that has come to be known as “The Black Muscle Bees.” These bees were discovered after processing a set of USDA-APHIS National Honey Bee Survey samples of adult honey bees. The samples of bees were crushed in water to prepare a solution that could be analyzed for Nosema sp., a fungal pathogen that is commonly detected in honey bee colonies across the country. The solution turned a dark shade of black, as opposed to the normal brown. This unusual observation warranted further investigation, so more bees were collected from multiple colonies in the same yard of the original “Black Bees.” Upon dissecting these bees it was discovered that the tissue inside of symptomatic bees was entirely black when compared to the standard pink tissue of healthy bees. It is thought that this darker color is caused by an increase in pigmentation or melanin formation. The melanin formation process is induced by a serine protease cascade involving the enzyme prophenyloxidase (PPO). The symptomatic “Black Bees” have a high level of PPO compared to asymptomatic bees from the same apiary. These bees were screened for other viruses and it was discovered that Deformed Wing Virus (DWV), a picornavirus, was more prevalent in the colonies with symptomatic bees when compared to healthy bees from the same yard. Additionally, the genetic diversity of these DWV strains was much higher in the colonies containing “Black Bees”. DWV is a virus transmitted by Varroa destructor, a parasitic mite that was introduced to the United States in the 1980s. Varroa has since been documented as a vector of a variety of honey bee viruses. It transmits viruses directly into the haemolymph, the insect equivalent of blood, of the bee as the mite feeds. This mode of inoculation bypasses the typical GI detoxification pathway of the honey bee. Varroa mites also provide these viruses with an additional place to replicate and diversify, ensuring that Dr. Boncristiani will have plenty of work to stay busy in his new field of honey bee virology. References: Boncristiani, H., Criado, M.F., Arruda, E., (2009). Respiratory Viruses. In M. Schaechter (Ed.), Encyclopedia of Microbiology (p. 4600). Academic Press. Cox-Foster, D. L.; Conlan, S.; Holmes, E. C.; Palacios, G.; Evans, J. D.; Moran, N. A.; Quan, P.-L.; Briese, T.; Hornig, M.; Geiser, D. M.; Martinson, V.; vanEngelsdorp, D.; Kalkstein, A. L.; Drysdale, A.; Hui, J.; Zhai, J.; Cui, L.; Hutchison, S. K.; Simons, J. F.; Egholm, M.; Pettis, J. S.; Lipkin, W. I. (2009). A Metagenomic Survey of Microbes in Honey Bee Colony Collapse Disorder. Science, 318, 283–287. Blog post written by: Olivia Bernauer is a first year Master’s student in Dennis vanEngelsdorp’s bee lab working with wild, native bees. Olivia is currently working with volunteers to monitor the floral preference of Maryland’s native pollinators. Andrew Garavito is a Master’s student in Dennis vanEngelsdorp’s Lab. He is studying honey bees, with a focus on the diversity of pollen types brought in by foragers, and the effects of different pollen diets on bee health. |
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290






 RSS Feed
RSS Feed




