|
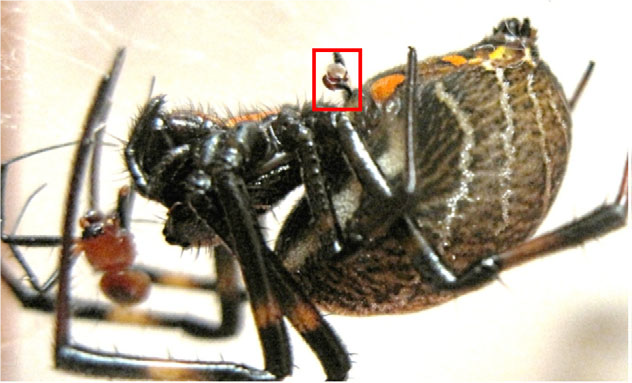
You have probably heard of at least one eight-legged femme fatale: the black widow. A female so fearsome she has inspired a multitude of book, movie and comic book characters. It turns out that this aggressive arachnid is not alone! Spiders are a group rich with outsized females. In biology, when one sex is differently sized than the other within the same species it is called sexual size dimorphism, or SSD. SSD can be male or female-biased, meaning either the male or female can be abnormally sized. Female-biased SSD, more common in egg-laying organisms, has evolved repeatedly in spiders. In fact, female-biased SSD has evolved four to nine times in the orb weaver group alone (Hormiga et al., 2000; Kuntner et al., 2015). Dr. Matjaž Kuntner, the Chair of the Biological Institute at the Research Centre of the Slovenian Academy of Sciences and Arts, studies the phenomenon of female-biased SSD in orb weaver spiders and has made several fascinating discoveries. He first became interested in SSD during his graduate fieldwork in the tropics while earning his doctorate at George Washington University. While studying orb weaver systematics in places such as Southeast Asia, Madagascar and South Africa, he witnessed the obvious size discrepancies between males and females. He fell into the UMD web when he took Dr. Jeff Shultz’s course, Arthropod Form and Function, in our own Department of Entomology.
Both behaviors indicate that Nephilids exhibit something called sexual conflict. This is when males and females have opposing goals and strategies during mating and can lead to the sexes evolving ever more complicated ways to outsmart and outmaneuver one another (Kuntner et al., 2015). While Dr. Kuntner’s lab works primarily on Nephilid behavior, the Argiopines also exhibit sexual cannibalism and emasculation, indicating sexual conflict could play an important role in this group as well (Nessler et al., 2009). One of Dr. Kuntner’s main goals is to understand the evolutionary patterns of female-biased SSD. In these systems, females are much larger than males, which begs the question: are females giants or are males dwarves? We assume that the ancestors of groups that exhibit SSD were once monomorphic, meaning males and females were the same size. Varying selection types and selection pressures lead to various iterations of SSD. Using analysis of evolutionary history, Dr. Kuntner was able to determine that the evolution of male and female body size is actually decoupled in Nephilids. Over time, females and males have both independently increased in size. However, females have undergone much greater increases over the same amount of evolutionary time. He termed this evolutionary pattern “sexually dimorphic gigantism.” Gigantism may evolve in Nephilid females in order to increase fecundity, or the number of eggs the female can carry (Lupše et al., 2016). In the Argiopines, while female and male size changes were positively correlated, there were no significant increases in body size. In fact, SSD appeared to decline over time. Dr. Kuntner also wanted to know if the evolution of body size was directional. He tested three evolution models: Brownian motion, directional evolution, and single optimum. In Nephilids, he found that size evolution of females was not directional and fell under the“Brownian motion” model, meaning the changes in size over time were random. Nephilid male size evolution,
Additionally, Dr. Kuntner has done a lot of work on the different morphological and behavioral phenotypes that relate to SSD. He found that complexity in male and female genitalia coevolve (Kuntner, Coddington et al., 2009). Kuntner’s group determined that as female Nephilids evolve to increase fecundity by growing larger and developing ways to mate with multiple males, males respond by developing ways to prevent females from mating with other males. A male’s reproductive fitness increases when he prevents a female from mating with other males by ensuring that she only bears his offspring. One such strategy, called “plugging,” occurs when males essentially castrate themselves and plug the female genitalia to keep other males from being able to mate with her. Other strategies include “mate binding,” which is when a male spins a web around a female to keep her
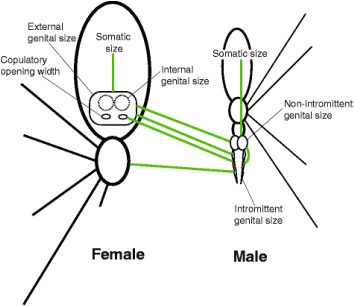
Dr. Kuntner’s more recent work has shifted from studying the evolutionary arms race between male and female orb weavers to looking at the perils of gigantism in female orb weavers. How big is too big? How is mating still possible with females that are so much larger than males? To answer these questions, Kuntner and his team measured hundreds of spider bodies and spider private parts (Lupše et al., 2016). They determined that female body size (or somatic size) evolved independently from internal genital size. Similarly, male body size evolved independently from intromittent (sperm delivering) genital size. However, male intromittent genital size is correlated with female external genital size which indicates that the evolution of these reproductive organs in males helps prevent genital size mismatches that would be expected as a result of SSD (Fig. 3). Dr. Kuntner plans to continue to explore the perils of gigantism in female orb weavers. There are many research questions that the mechanism of SSD poses, such as how gravity affects these large female spiders and how they have had to alter the web designs and functionality to compensate for increases in size. About the Authors: Grace Anderson is a Master’s student in the Shultz Lab at in the UMD Department of Entomology. Her work focuses on the evolution, behavior and systematics of harvestmen. She also is the Assistant Director of the Entomology Department’s Bug Camp. Andrew Garavito is a Master’s student in Dennis vanEngelsdorp’s Lab. He is studying honey bees, with a focus on how pollen consumption impacts the ability of bees to fight off infections. References: Cheng, R.C. & Kuntner, M. 2014. Phylogeny suggests non-directional and isometric evolution of sexual size dimorphism in argiopine spiders. Evolution. doi:10.1111/evo.12504 Hormiga, G., Scharff, N., & Coddington, J. A. (2000). The phylogenetic basis of sexual size dimorphism in orb-weaving spiders (Araneae, Orbiculariae). Systematic Biology, 49(3), 435-462. Kuntner, M., Agnarsson, I. & Daiqin Li. 2015. The eunuch phenomenon: adaptive evolution of genital emasculation in sexually dimorphic spiders. Biological reviews 90: 279–296. Kuntner, M., Arnedo, M.A., Trontelj, P., Lokovšek, T. & Agnarsson, I. 2013. A molecular phylogeny of nephilid spiders: Evolutionary history of a model lineage. Molecular Phylogenetics and Evolution 69: 961–979. Kuntner, M. & Cheng, R.C. 2016. Evolutionary pathways maintaining extreme female-biased sexual size dimorphism: Convergent spider cases defy common patterns. In: Pontarotti, P. (ed) Evolutionary biology: Convergent evolution, evolution of complex traits, concepts and methods. Springer International Publishing, Cham, pp 121–133. Kuntner, M., Coddington, J. A. & Schneider, J. M. 2009. Intersexual arms race? Genital coevolution in nephilid spiders (Araneae, Nephilidae). Evolution 63: 1451-1463. Kuntner, M., Kralj-Fišer, S., Schneider, J. M. & Li, D. 2009. Mate plugging via genital mutilation in nephilid spiders: an evolutionary hypothesis. Journal of Zoology 277: 257-266. Kuntner, M. & Elgar, M. A. 2014. Evolution and maintenance of sexual size dimorphism: Aligning phylogenetic and experimental evidence. Frontiers in Ecology and Evolution 2: 26. Li, D., Oh, J., Kralj-Fišer, S. & Kuntner M. 2012. Remote copulation: male adaptation to female cannibalism. Biology letters 10.1098/rsbl.2011.1202 Lupše, N., Cheng, R.C. & Kuntner, M. 2016. Coevolution of female and male genital components to avoid genital size mismatches in sexually dimorphic spiders. BMC Evolutionary Biology 16:161 Nessler, S. H., Uhl, G., & Schneider, J. M. (2009). Sexual cannibalism facilitates genital damage in Argiope lobata (Araneae: Araneidae). Behavioral Ecology and Sociobiology, 63(3), 355-362. Aliens are invading the forests of the United States! Not the green, bug-eyed aliens from outer space; no we are talking about the, well… green, bug-eyed aliens from Earth. With the globalization of trade, insect introductions leading to invasive pest problems have steadily increased over the last few centuries, causing massive economic and environmental devastation in the systems where these pests permeate. These invaders are especially difficult to manage when they are pests of our native North American forest trees due to the large spatial scale associated with them, making pesticide applications impractical.
having a warmer climate than Connecticut, created an asynchronous relationship between the host (EHS) and the parasitoid (E. citrina) in Connecticut. This means that the scale and parasitoid are developing at different times of year, preventing the wasp from being able to effectively attack the scale in its introduced range. With the colder climate of Connecticut, it was hypothesized that the EHS scales developed more slowly. Wasps, as a result, would have fewer suitable 2nd instar hosts to parasitize. Dr. Abell tested this by observing scale abundance and parasitism by E. citrina at three distinct latitudes in the U.S. (Connecticut [“coldest”], Pennsylvania, North Carolina [“warmest”]), hypothesizing that he would find more parasitoid-host synchrony as he moved further south where warmer temperatures would allow for multiple generations of scales. Ultimately, Dr. Abell did not observe any increase in synchrony between EHS and E. citrina at any of his three field sites. Instead he found continuous reproduction of EHS, and all life stages were present throughout the year. This led Dr. Abell to Japan to better understand how EHS behaves in its native range. While surveying hemlock scales and their associated parasitoids, Dr. Abell found 11 new species attacking EHS in Japan, some of which may have potential as classical biological control agents.
a wasp that is less than 1mm in length that attacks EAB eggs. Research done by Duan et al. in 2013 indicated that T. planipennisi was effectively established in Michigan and is a strong disperser. However, they observed that there was no parasitism of EAB in larger trees. In a study done by Dr. Abell, it was determined that the bark thickness was preventing this small wasp from attacking the EAB larvae. The ovipositor (egg-laying mechanism) of T. planipennisi is too short to reach the EAB larvae underneath the thick bark.
The bark was also placed in emergence chambers to collect any parasitoid wasps that emerged from the bark remnants that were missed in earlier screening. After two years of testing these methods, Dr. Abell concluded that the bark-sifting method was a more effective way to measure the rate of O. agrili egg parasitism in the field because significantly more parasitoids were recovered with this method. Invasive insects continue to attack our forests today, therefore it is very important to continue to understand and utilize biological control methods to preserve our forests. Dr. Abell continues his work on EAB biological control in the Shrewsbury lab here at the University of Maryland where he is evaluating other introduced and native parasitoids and additionally an integrated approach that combines pesticides with classical biological control methods.
About the Authors: Olivia Bernauer is a second year Master’s student in Dennis vanEngelsdorp’s bee lab working to better understand the floral preferences of Maryland’s wild, native pollinators. Jackie Hoban is a second year Master’s student working on emerald ash borer biological control in Paula Shrewsbury’s lab. On Tuesday, October 18 UMD's Division of Research presented the winners of its 2nd Annual Research Communicator Impact Award at the 18th Annual Research Leader's Luncheon. The offering of this award began in 2015 with the intent of nominating researchers who take extra steps to share their research, opinions, and knowledge with the public though media means such as self-created videos, blogs, and news outlets.
The Department of Entomology's very own Dr. Mike Raupp was one of four recipients for the award. He won for his popular and engaging Bug of the Week blog and YouTube channel which engages the global community with the insect wonders found in Maryland. More than 30 faculty members were nominated for the award across 12 different colleges and schools within the University. Kimberly Nesci, a 1996 entomology graduate and former graduate student of Dr. Galen Dively has provided over 20 years of excellent services in the world of pesticide regulation, 17 of which she worked directly in the Office of Pesticide Programs for the Environmental Protection Agency. Now, Nesci has advanced further into the pesticide sphere, accepting the position as the acting associate director for the Environmental Fate and Effects Division within the EPA.
Nesci graduated from University of Maryland with a Master of Science in Entomology. Her career started at the EPA’s Office of Pesticides as a Chemical Review Manager. A short four-years later, Nesci became a team leader in SRRD contributing her expertise to organophosphate reregistration actions/mitigations and the cancellation of lindane (a formerly common pesticide revealed to cause neurotoxic effects). After these accomplishments, Nesci joined the Registration Division as a Product Manager in the Insecticide Branch where she worked on the pet spot-on mitigation efforts, an intricate plan produced by the US-Canada Regulatory Cooperation Council to assess the risks of insect treatments on pets. Simultaneously, she served as a member of and chaired meetings for the Food and Agriculture Organization’s Panel of Experts on Pesticide Management, a group that initiated best management practices for pesticide application in developing countries. Her resume continued to grow thereafter. Beginning in 2013, Nesci has served as Chief of the Microbial Pesticides Branch in the EPA’s Biopesticides and Pollution Prevention Division, in which she was responsible for the scientific evaluation and constructing regulations for microbial pesticides and Plant-Incorporated Protectants. Her multi-disciplinary work in this position made her an influential component on the policy making, science, and regulatory decisions on new RNA-interference-based pesticide technologies and the development of resistance to certain proteins in genetically engineered crops. On September 19, 2016 Nesci ascended once more within the EPA by accepting a position as the acting Associate Director for the EPA’s Environmental Fate and Effects Division. The Department of Entomology is sending our best wishes to this astounding member of our alumni community. We are proud of what this Terp has accomplished and what she will continue to achieve well into the future. This past Saturday, members of the Department of Entomology heading on over to the College of Agriculture and Natural Resources (AGNR) Open House at the Central Maryland Research and Education Center in Ellicott City. The event lasted from 10 AM until 3 PM, where we engaged with many curious visitors, providing them with a wealth of information about our outstanding programs and the culmination of academic, research, and extension resources available to our students. In addition, guests got to participate in bird-watching, farm tours, interactive displays, and indulge in delicious treats from student organized food tents. Thank you to everyone who helped and attended! We hope to see you at UMD soon with all your FEARLESS IDEAS!
What makes a species a species? What are the characteristics that make it unique enough to be distinguished from similar creatures? What are the genetic underpinnings that allow species to evolve to create a distinct entity? These are the type of questions Dr. Carlos Machado is trying to answer. Answering them are key to understanding our planet's biodiversity. A traditional definition of speciation centers around the concept of reproductive isolation. While members of two different species may mate, if they either produce no offspring or sterile offspring, these two individuals represent different species. This is known as the biological species concept. However, what factors lead to reproductive isolation? One way is through physical separation of populations (e.g., a mountain range) or allopatric speciation. On the other hand, what about sympatric speciation, or speciation in overlapping geographical distributions?
Dr. Machado’s work brings us a step closer to understanding the roots of speciation. Following from this work, his future research ideas center on correcting inversions using the latest genetic techniques, such as CRISPR (Clustered regularly interspaced short palindromic repeats), to study in further detail these regions that are responsible for species’ differences. Jonathan Wang is a PhD student in Raymond St. Leger’s lab studying host-pathogen interactions. Jen Jones is a PhD student in Bill Lamp’s lab, studying how socioeconomic factors influence the distribution of mosquito populations. As the world’s population continues to grow, a key issue facing society is how to balance feeding the world while protecting the environment. Dr. Kate Tully has worked on this problem in both East Africa and the United States, where the solution will require different approaches, in part due to the different use of crops. In developed nations, many grain crops are grown for animal feed and fuel compared to developing nations where most are grown for human consumption. As such, different goals and approaches need to be taken for a sustainable future. While crop yield has increased in many parts of world since the 1960s, this trend has not occurred in sub-Saharan Africa. Dr. Tully is working in Kenya and Tanzania to determine what changes can improve yield without excessive fertilizer use. Africa as a whole plans to increase nitrogen-based fertilizer use, but there is an upper limit to the amount plants can use before the rest is lost to the environment.
Large amounts of fertilizer nitrate were leached through the sandy soils to groundwater. Dr. Tully concluded that, in addition to using better overall management practices such as irrigation and increasing organic matter content, site characteristics should determine the optimal nitrogen fertilizer amount; in these cases, farmers in Kenya can increase yield by adding 75-100 kg N/ha while those in Tanzania should only use 50 kg N/ha. These levels can increase yield while protecting the environment from excess nitrogen leaching into waterways and gassing off into the air. Back in the U.S., Dr. Tully turned her attention to soil nutrient dynamics on the Eastern Shore, Maryland. The dominant agricultural crops feed—corn and soybeans support their primary industry—poultry. Over the years, phosphorus from fertilizers and chicken litter has accumulated in the soil to the point of saturation. Phosphorus typically binds to soil particles with minimal nutrient leaching to the surrounding environment. Yet, as human-accelerated sea-level rise causes saltwater intrusion of coastal farmland, soil dynamics are changing. Saltwater can significantly alter soil chemistry, which may result in the release of bound soil nutrients as runoff. While nitrogen and phosphorus are essential nutrients for living organisms, too much can throw entire ecosystems off balance. Excess phosphorus in particular has led to algal blooms in fresh water estuaries. Over time algal blooms deplete life supporting oxygen resulting in ‘dead zones.’ But to what magnitude, rate, and extent are saltwater intrusions increasing the movement of phosphorus?
Read more of Dr. Tully’s work!
Tully K, Hickman J, McKenna M, Palm CA, Neill C. 2016. Effects of increased fertilizer application on inorganic soil N in East African maize systems: vertical distributions and temporal dynamics. Ecological Applications 26: 1907-1919. doi:10.1890/15-1518.1 Becca Eckert is a Ph.D. student in Bill Lamp’s lab. Her research examines how changes in light and nutrient availability affect macroinvertebrate growth and diversity in heterotrophic headwater streams as mediated by changes in leaf-associated algal quantity and quality. Lisa Kuder is a Ph.D. student in Dennis vanEngelsdorp’s Bee Lab. Her research focuses on road ecology, specifically improving highway rights-of-way for pollinators. |
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290












 RSS Feed
RSS Feed




