|
Post by Lauren Hunt and Thomas Pike On 22 November 2013, we heard MS degree candidate Ashley Jones give her exit seminar in Entomology Colloquium, describing research on indigenous natural enemies of the brown marmorated stink bug (BMSB). BMSB is an invasive species from Asia that arrived in Allentown, PA in 1996 and spread quickly. Now recorded from 40 states, BMBS is an extraordinarily destructive pest of both ornamentals and food crops primarily due to their extremely polyphagous nature. Originally considered merely a nuisance pest, in 2010 BMSB populations exploded, prompting farmers and researchers alike to take action to halt the spread. Biological control is an attractive option, as there are many egg parasitoids and predators in the stink bug’s native range. The goal of Ashley’s research was to determine baseline natural enemy behavior in the US in the hopes that natural enemy complexes could be conserved and promoted to control BMSB. Ashley’s talk focused primarily on the native egg parasitoids of BMSB. During the 2012 and 2013 field seasons, several ornamental tree genera (Acer, Prunus and Ulmus) were checked weekly for eggs. These eggs were then monitored for mortality and parasitoid emergence, which can be seen in this video. Egg mortality was categorized as follows: no mortality, chewing mortality, sucking mortality, parasitism or unascribed mortality (cause unknown). In both seasons, parasitism was the primary source of mortality by a wide margin (32% parasitism in 2012, 44% in 2013). This parasitism increased during the season and was primarily the work of a single genus, Anastatus, which was responsible for 98% of the egg parasitism. Perhaps most intriguing was the change in sex ratio from 2012 to 2013. In 2012, Anastatus reduvii (the most prolific egg parasitoid) had a male:female ratio of 1:2.05. In 2013, this ratio jumped to 1:4.85. Ashley posits that this is most likely due to an increase in eggs available in 2013, encouraging the parasitoids to produce more females to exploit this resource. Ashley concluded that predation by various common nursery predators did seem to augment control of the BMSB. She observed a distinct pattern in the way the predator density and the stink bug infestation were related. As the number of stink bugs decreased in the season, so did the number of predators observed. Likewise, as the number of stink bugs present increased, the number of predators rose with slight delay, compensating, it seems, for the increase in food availability. This pattern was consistent in both years she ran the study. In the laboratory, Ashley used feeding trials to determine which natural enemies were the most successful at control. She conducted trials on wheel bugs, jumping spiders, lacewings, minute pirate bugs, convergent lady beetles, and multicolored Asian lady beetles. Across all life stages of BMSB, wheel bugs were most voracious by far. The lab BMSB egg trials, however, did not show what was clearly demonstrated in field observations. No predators consumed the eggs in these trials, even though lacewing larvae had been observed feeding on BMSB eggs throughout the summer. This may be attributed to a different species of lacewing used in lab trials compared to what is found in the field setting. Remaining unanswered and open for additional investigation is the occurrence of unascribed mortality, in which the BMSB eggs did not hatch and died, with causes unknown. Could this arise from feeding by adult parasitoids? A fungal pathogen, perhaps? A byproduct of a feeding predator? Because unascribed mortality was more common with larger egg clutch sizes (density dependent), this tends to favor a biotic hypothesis over, for instance, extreme temperature, the effects of which should not differ with egg density. The more information we have, the more possible research avenues become available to learn how to best control these pests in an environmentally safe and sustainable manner. Ashley suggested further research is needed on the main species of parasitoid found successfully controlling BMSB in ornamentals and nurseries, Anastatus reduvii. Although we know Anastatus is a generalist parasitoid, we know relatively little about other factors that may directly influence its longevity and fecundity. By gathering more information about this promising biological control agent, it might be included as a component of an Integrated Pest Management (IPM) program to control these pesky stinkers in our nurseries, gardens, and homes. About the authors
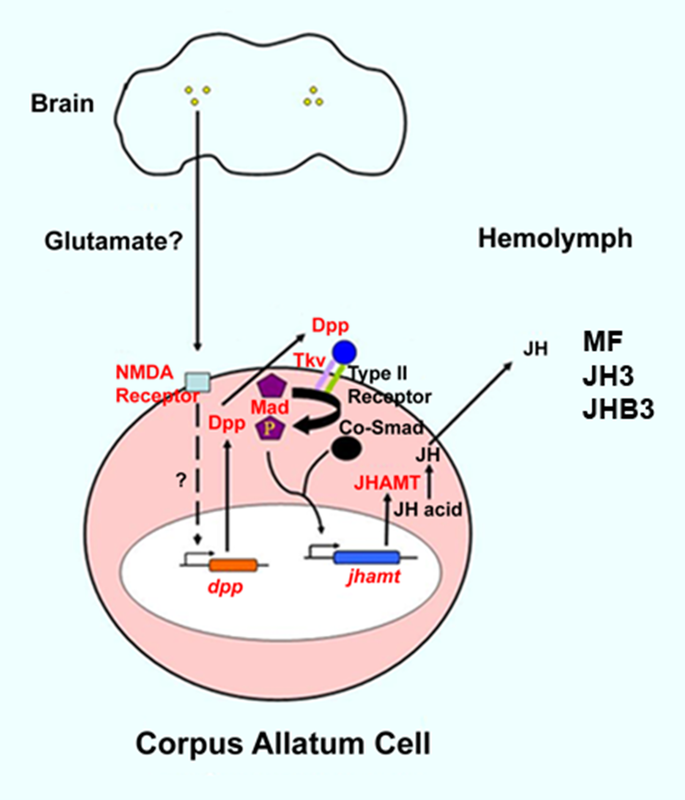
Lauren Hunt is a first year Master’s student conducting research focused on biological control of stink bugs, with emphasis on the usage of insectary plants to increase mortality of the invasive brown marmorated stink bug (BMSB). With exponential human population growth, there is an intensifying need for all varieties of sustainable living practices, including food and grain production. She is interested in the development of techniques that will conserve natural resources and biological living systems. Thomas Pike is a second year Master's student studying the effects of entomopathogenic fungi on the brown mamorated stink bug with regards to its use as a formulated biocontrol. He is also pursuing trap-and-kill systems utilizing BMSB pheromones and the fungus to be used as a passive control. These control methods show promise as an alternative to current pesticide use in ornamental and crop systems. The generation of a mature insect from its larval stage might seem unimaginable at first. For instance, how can a worm-like larva transform into a winged butterfly? They are completely different, not only in their appearance but their physiology and behavior as well. With the correct cellular and molecular mechanisms, however, the entire process of metamorphosis can be properly coordinated, often in a swift and timely manner. This, and questions concerning the details of this phenomenon, is precisely the subject of study for Dr. Jian Wang. After receiving degrees at Nanjing Agricultural University andShanghai Institute of Entomology, Dr. Wang went ahead to investigate the aforementioned topic, with a concentration specifically in the laboratory fruit fly, known as Drosophila melanogaster. The “lab rat” Drosophila, while a useful genetic model, can also serve as a model for insect physiology. Thus, this animal can provide crucial information on the function of genes and signaling pathways that are often, and conveniently, transferrable to their vertebrate counterparts. At the same time, scientists who study Drosophila can apply novel information learned about insect hormonal changes, reproduction, behavior etc. to insects at large, thereby providing a centralized method for studying these invertebrates as a whole. 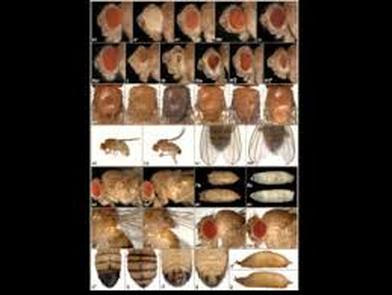 The image above is a compilation of just a few different genotypes/phenotypes of Drosophila; the specialized field of uncovering what different genes do, many times by creating mutations and observing the resulting phenotypes, is known as functional genetics/genomics. (http://www.drosophila-images.org/2006.shtml) One of the main questions that Dr. Wang is interested in solving is the pathway of Juvenile Hormone (JH) signaling, including the identification of signaling molecules and receptors that compose this pathway. Initial evidence showed that the ablation, or gross removal, of a neuroendocrine structure known as the corpus allata (CA) resulted in pupae that would die prematurely before they could emerge as adults. Thus, the CA was identified as a major determinant in insect metamorphosis. Next, a series of experiments showed that when the CA was destroyed, it was possible to partially rescue the lethal developmental effects by adding any of 4 different “Juvenile Hormones”, 3 naturally-occurring hormones and the other a well-known artificial analog of this hormone. Eventually, a complete picture was formulated for the juvenile hormone pathway: the brain stimulates the cells of the CA with neurotransmitters to produce dpp, a morphogen involved in development; and this molecule activates the TGF-β signaling pathway to produce JHAMT, an enzyme involved in the synthesis of any of three juvenile hormone isoforms. The other main topic of Dr. Wang’s research is the function of genes that are important to the development and maintenance of the nervous system. Much of this work uses a genetic mosaic technique, which can generate parts of an animal that are completely mutant for a particular gene. One well-studied brain structure in the Wang lab is the mushroom body. Critical to formation and retrieval of olfactory memory, the mushroom body is a structure composed of bundles of dendrites and axons. Ubiquitous in insects, and possessed by some non-insects as well, this suborgan has a characteristic V-shaped axonal branching pattern. The degeneration and/or malformation of these axonal lobes has been under intense study in the Wang lab, whereby the function of a gene can be deduced by the aberrant resultant phenotype observed following the generation of a mutation for that mushroom-body expressing gene. Of particular interest in the past decade or so was the function of DSCAM, a protein involved in neuron synaptic formation, the precise location where two neurons meet and communicate at the molecular level. A multitude of mutations were generated for the gene producing this protein which resulted in two important discoveries. Firstly, a DNA sequence analysis showed that the Drosophila gene producing DSCAM is conserved, in terms of nucleotide base pairs, with a gene found in humans. Secondly, through a clever transgenic assay, it was found that pupation rate, and to a lesser extent eclosion rate, could be partially rescued by overexpressing different forms of human DSCAM in mutant flies unable to produce any DSCAM of their own. These two experiments provided evidence that human and Drosophila DSCAMs not only have a conserved sequence, and are possibly evolutionarily related, but that they are functionally conserved as well. Currently, the Wang Lab is continuing its quest to uncover more genes that play a critical role in the development of the Drosophila nervous system, using the mushroom body as the experimental tissue of choice. The senior graduate student, Lijuan Du, is currently investigating the overlap between the Hippo pathway and the JAK/STAT pathway. Over the years, she has found evidence that one particular gene is regulated downstream by both of these pathways. These pathways are crucial in regulating the cell cycle, carefully guiding cells through proper rates of cell proliferation and programmed cell death. The other member and writer of this blog, Justin Rosenthal, is beginning his second year in the Wang Lab. The focus of his research is the function of a single gene, Darkener of apricot(Doa), in ensuring neuronal viability through the drastic changes of metamorphosis. He has already confirmed work done by previous students that this gene is necessary for survival of the y-lobe of the mushroom body and is now devising experiments to test the role of the individual variants of this gene and the functional importance of some of the exons, or the coding parts of the gene. 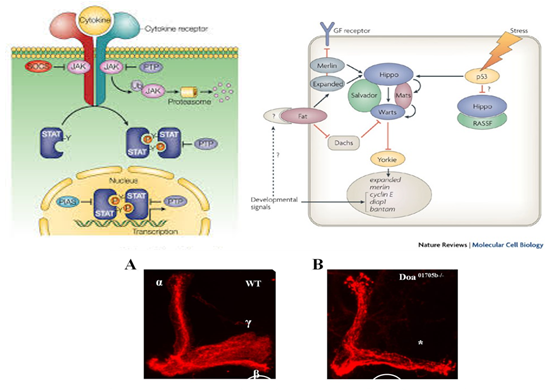 The two top images show the JAK/STAT (left) and hippo(left) pathways, delineating the intricacies of each. The bottom photo is of two mushroom bodies, visualized with a red fluorescent marker. The left is the wt condition and the right it the mutant condition. One can easily see the stark differences, wherein the mutant mushroom body is completely missing the medial-branching y-lobe(top left: http://www.nature.com/nri/journal/v3/n11/full/nri1226.html), (top right: http://www.nature.com/nrm/journal/v8/n8/fig_tab/nrm2221_F3.html) (bottom: Qiong Yao) Article published by Dr. Wang and others: http://www.sciencedirect.com/science/article/pii/S096517481100110X Article related to neuron patterns in Drosophila:http://www.nature.com/nature/journal/v497/n7447/full/nature12063.html About Justin:
Justin Rosenthal received his undergraduate degree from the University of Maryland-College Park in 2011 in the Biological Sciences, with a concentration in neurobiology/physiology. Upon beginning his PhD. Program here, Justin began investigating the role of a particular gene, darkener of apricot(Doa), in promoting neuron survival through the pupal stage of insect life, i.e. metamorphosis. Building upon previous research, it became ever more convincing that without this gene certain neurons within aDrosophila’s brain will not survive until adulthood. Currently he is working out the purpose of specific exons and isoforms of this gene, as several variations exist. Further research will likely include expansion of this investigation into other non-nervous tissue. Overall this information will provide a molecular model for how cell death, especially in neurons, proceeds. Post by Samuel Ramsey and Rebecca Wilson In previous colloquia this semester, we’ve had the pleasure of learning about research focusing on a variety of different arthropods, ranging from potato leafhoppers to harvestmen. However, our most recent colloquium deviated slightly with a presentation focusing on entomopathogenic fungi and their potential to control the spread of deadly vector-borne illnesses. Dr. Raymond St. Leger started his presentation in the most well attended colloquium of the semester thus far with a video showcasing a diverse array of stunningly bizarre Cordyceps fungi. Many are known to have a profound and often grotesque effect on their infected insect hosts, with some well known for influencing insect behavior. Entomopathogenic fungi boast great promise as biological control agents due in no small part to their strong host-specificity and staggering diversity. Dr. St. Leger explained that are species of fungi that specialize in killing basically every species of insect. This list includes the mosquitoes responsible for vectoring malaria, a disease that causes more than 1 million human deaths annually. As the mosquito and its Plasmodium parasite is the cause of inimitable human suffering, help from this fungus is entirely welcome. Unfortunately, Cordyceps prefer to grow inside of their host insect, making them rather difficult to culture in labs. The St. Leger lab has circumvented this issue by working with two anamorphs of Cordyceps, Beauveria and Metarhizium. They have lost their sexual phase, and in so doing, are a lot less temperamental. Metarhizium, which occurs frequently in nature as a plant symbiont, initially showed promise as a biological control agent against mosquitoes, but lacked the necessary virulence to kill them quickly enough to prevent the many bites that spread malaria. As St. Leger explained to us, it is not naturally in a pathogen’s interest to exterminate its host species. Mosquitoes infected with Metarhizium, in many cases, were able to feed and even mate before succumbing to their fungal parasite. By super-charging these fungi with proteases, the St. Leger lab was able to significantly decrease the amount of time required to kill mosquitoes and suppressed their appetites, in the interim, even in the presence of potential hosts. Another undertaking in the lab involves producing a fungus that is able to kill the causative agent of malaria inside of the mosquito’s hemolymph and preventing its spread into the salivary glands, thereby curing the mosquito to render her harmless upon her next blood meal. Clearly, the St. Leger Lab has been busy, as they were able to transgenically express a scorpion toxin and that of a funnel web spider in the fungus to greatly increase its virulence. Dr. St. Leger and his graduate students are also involved with developing fungi that can remedy other insect related human health and welfare issues, including famine. They’ve worked with developing and mass-producing strains of Metarhizium that may reduce populations of locusts and coffee borer beetles. Research on this astounding group of fungi and their use in biological control is currently being advanced worldwide. It was great to learn that our own Dr. Raymond St. Leger continues to work at the forefront of this vital field. Fang W, Vega-Rodriguez, J., Ghosh, A.K., Jacobs-Lorena, M., Khang, A and St. Leger, R.J., 2011. Development of transgenic fungi that kill human malaria parasites in mosquitoes Science 331: 1074-1077. Samuel Ramsey is a 2nd year PhD student in the Shrewsbury lab currently studying competition in egg parasitoids of the brown marmorated stink bug.
Rebecca Wilson is a 1st year master’s student in the lab of Dr. Bill Lamp. She is broadly interested in aquatic entomology and is currently studying larval black fly distribution in western Maryland. Last week, our department welcomed visiting scientist, Dr. Nora Underwood to present her lab’s research at our weekly colloquium seminar. And although Dr. Underwood hails from Florida State University, whose football team defeated the Terps 63-0 in a record setting game a few short weeks ago, she was warmly received by a packed house here at the University of Maryland. This is surely due, in no small part, to Dr. Underwood’s connection to two of our own who came to us from her lab. Graduate student, Alex Forde (Gruner Lab) and post-doc Amanda Buchanan (Hooks Lab) both hail from the Underwood lab as former lab tech and graduate student, respectively. However, the real draw for most of the crowd was surely the topic of plant-insect interactions, which is an area of research shared by many labs within our own department. Dr. Underwood’s presentation came to us in two parts, and although there was an admission of disconnect between the topics, there is one theme that bridges the divide, and that is an addiction to pursuing questions with huge, field-based studies. The first half was a discussion of a large study exploring mechanisms of changes to a plant population resulting from damage inflicted by insect herbivores. Every farmer, gardener, and even grad student knows that plants don’t grow too well when insects are eating them. However, this study seemed to stem from being dissatisfied with simply acknowledging that insect herbivores are bad for plant populations, and the need to know exact reasons why insect herbivory might cause plant populations to decline. To address these questions, Dr. Underwood and colleagues experimented with populations of horsenettle (Solanum carolinense), measuring growth, fecundity, asexual reproduction, and rates of herbivory over two years for each individual plant, all while manipulating plant densities and herbivore abundances. The idea behind measuring all of these demographic factors rather than simply measuring a change in biomass was to get at how and not just whether insect damage leads to change in the population. For example, a perennial plant population may decline from one year to the next because some plants were killed, because there were fewer energy reserves in the roots of overwintering plants, because there was decreased seed production, or because there were fewer “runners” produced asexually. To be able to answer these questions, though, requires compiling a massive dataset of all these demographic factors, and then using stage-structured demographic models to show how each factor is affected by different levels of herbivory. For more information on this study, see Dr. Underwood’s publication in the journal Ecology (Underwood & Halpern 2012). 
Dr. Nora Underwood’s research involves following the growth and development of each individual in populations of this plant, Carolina horsenettle (Solanum carolinense), in order to learn about how effects of plant density and insect herbivory interact. Photo Credit: Ted Bodner, Southern Weed Science Society, Bugwood.org
The second half of the talk introduced a new framework for studying associational effects within plant communities. Associational effects account for how the struggle for plants to avoid being eaten by insect herbivores occurs within a complex matrix of other species of plants, and that plant insect interactions involve more than just the interaction of the insect and the focal plant. In other words, if you are a plant, how susceptible you are to herbivores may depend on who your neighbors are? The effects that neighboring plants can have on the species in focus have been described many different ways. For example, neighboring plants may produce volatile chemicals that limit an insect’s ability to find the focal plant, which would result in lower herbivory rates. On the other hand, neighboring plants may serve as a host to many species of herbivores that also feed on the focus plant species, making it more susceptible to attack from herbivores. Dr. Underwood presented a novel framework for studying these kinds of effects on herbivory rates between neighboring plant species as a way to tease apart mechanisms underlying the effects. In doing so, Dr. Underwood has also signed up her lab for even more large, field based experiments, because to differentiate between effects of density of the focal species and density of the neighboring species, both have to be changed simultaneously. Fortunately, horsenettle is probably more than willing to oblige, as it is a noxious weed throughout much of the United States. This fact also helps the Underwood lab to walk the thin line between basic and applied ecological research, as the results of these experiments are useful not only from the standpoint of our basic understanding of how plants and insects interact in nature, but in informing us of ways to manipulate the landscape to reduce damage to focal species like crops, or ornamentals. More information in this new framework can be found in a new publication by Dr. Underwood and others, which will be out some time next year (Underwood et al. 2014). Underwood, N. and S. Halpern. 2012. Insect herbivores, density dependence, and the performance of the perennial herbSolanum carolinense. Ecology 93(5): 1026-1035. Underwood, N., B.D. Inouye and P.A. Hambäck. 2014. A conceptual framework for associational effects: when do neighbors matter and how would we know? Quarterly Review of Biology: in press. About Alan:
Alan Leslie is a Ph.D. candidate in the Lamp Lab, studying aquatic macroinvertebrates and their effects in regulating ecosystem functions. His research project is focused on determining the effect that burrowing aquatic invertebrates have on nutrient transport in agricultural drainage networks. |
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290





 RSS Feed
RSS Feed




