Fall 2015 Colloquium: Dr. George Dimopoulos of John Hopkins University School of Public Health12/21/2015
A Better Mosquito Malaria is a poorly named disease: literally meaning, “bad air,” it was born out of the miasma theory that disease was spread not by germs, but by a pollution of the air. Today, we know that it is mosquitoes, flying through the air, which spread this disease in a delicate dance between parasite (Plasmodium), vector (Anopheles mosquitoes) and host (humans). Malaria has been killing us since the dawn of civilization, so scientists are hard at work to throw this dance off-kilter. The most obvious solution is the one you implement with your hand whenever a mosquito lands on you: kill the mosquito. As a result, much of the work in vector biology aims to cut in on the dance between mosquitoes and humans; however, during the UMD Entomology colloquium this week, Dr. George Dimopoulos of Johns Hopkins University explained how he is trying to tip the scales where the mosquito and the parasite interact. 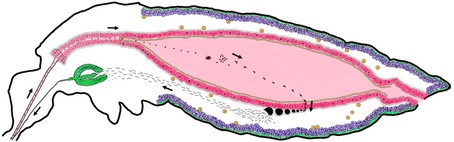 Figure 1: The developmental cycle of the malaria parasite in a mosquito. In this cross section of a mosquito, we can see how the parasites enter the mosquito through the proboscis (mouth) and imbed into the gut (pictured center, pink). After developing, the parasites burst into the mosquito blood and enter into the (green) salivary glands. From there, they are injected into a human host during biting. Image: Dimopoulos group. Figure 1: The developmental cycle of the malaria parasite in a mosquito. In this cross section of a mosquito, we can see how the parasites enter the mosquito through the proboscis (mouth) and imbed into the gut (pictured center, pink). After developing, the parasites burst into the mosquito blood and enter into the (green) salivary glands. From there, they are injected into a human host during biting. Image: Dimopoulos group. When Dimopoulos began investigating mosquito immunity, many found such work interesting, but impractical. Few could anticipate the exponential growth of the fields of molecular biology and genetics: in just 20 years, the number of known genes in a mosquito went from 13 to about 14,000. Scientists can now finely rewire the genes of insects, allowing Dimopoulos and his colleagues to “build a better mosquito:” one which does not carry the malaria parasite. In order to develop a better mosquito, it is important to keep in mind that the mosquito immune system is working with us to prevent malaria. While wild mosquitoes may ingest and spread many malaria parasites, the number of surviving parasites diminishes into the single digits in the gut of the mosquito (Figure 1). This bottleneck effect leaves the parasite vulnerable because at this point, the death of a few parasites in each mosquito would be the end of malaria. A mosquito may consume numerous parasites, but only end up with a couple surviving in its gut because its immune system fights hard to kill every last one. With the mosquito immune system as his ally, Dimopoulos engineered mosquitoes armed with enhanced immune systems able to annihilate invading parasites. He did this by combining genes already present in the mosquito. He increased the expression of a regulatory gene (Rel-2) from the IMD pathway (named the “Immune Deficiency” pathway because mosquitoes without this crucial pathway are susceptible to many pathogens), which turns on expression of multiple immune genes at the end of this pathway. By using a regulatory gene to attack the parasites on all fronts, Dimopoulos describes this method as similar to a “multi-drug therapy, by targeting the pathogen by multiple drugs; perhaps lessening the pathogen’s ability to evolve resistance.” He chose to combine this gene with the promoters (which determine when a gene is expressed) from two other genes: a protease (carboxypeptidase) or an egg production gene (vitellogenin). These genes are very highly expressed when a mosquito consumes blood, which is when the parasites enter the gut. This is also just before the bottleneck. These combinations of genes supercharge the immune system when the mosquito needs it most [1]. When Dimopoulos feeds these mosquitoes Plasmodium in the lab, the majority can successfully prevent the malaria infection. However, for this technique to work in the field, the Dimopoulos group wants to prevent infections entirely in all of the mosquitoes. To do this, they are creating hybrid strains expressing both the egg production and protease gene. They are also planning to try combinations with other immune genes to further enhance the immune system and bring infection down to zero. 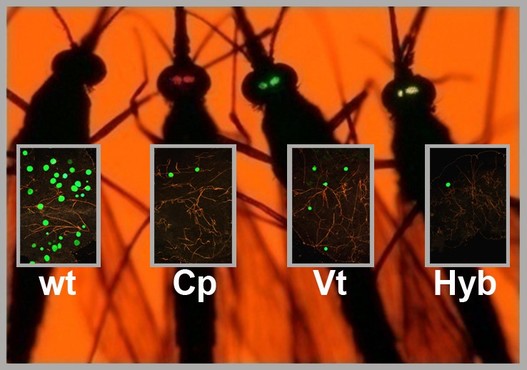 Figure 2: Four mosquitoes demonstrating their susceptibility to Plasmodium. Wt is an unmodified mosquito, Cp expresses Rel-2 driven by the carboxypeptidase promoter (red eyes), Vt has Rel-2 driven by the vitellogenin promoter (green eyes), and Hyb is a hybrid of both Cp and Vt. The green spots in the boxes over each mosquito represent their level of Plasmodium infection. Image: Dimopoulos group. Figure 2: Four mosquitoes demonstrating their susceptibility to Plasmodium. Wt is an unmodified mosquito, Cp expresses Rel-2 driven by the carboxypeptidase promoter (red eyes), Vt has Rel-2 driven by the vitellogenin promoter (green eyes), and Hyb is a hybrid of both Cp and Vt. The green spots in the boxes over each mosquito represent their level of Plasmodium infection. Image: Dimopoulos group. In lab experiments, Dimopoulos has found that his mosquitoes can gain resistance to malaria without a fitness cost, challenging the “dogma that says that it is impossible to make something fitter than an organism in nature.” He theorized that this is because the burst of immune system activity is limited to the time when blood is ingested; this happens to be a period of energy abundance for mosquitoes as they rest and digest their blood meal. Dimopoulos stressed the need to validate all his lab findings in field conditions at the Johns Hopkins MalariaSphere in Zambia. Recently, “the overall field of transgenic mosquitoes for control of vector borne diseases has received new enthusiasm” due to significant developments in biotechnology (such as novel gene drive systems). We have entered a translational phase where ideas and knowledge gained from basic research get applied, and when it comes to vector control involving mosquito immunity, Dimopoulos is leading the way. References:
1. Dong, Y., Das, S., Cirimotich, C., Souza-Neto, J. A., McLean, K. J., & Dimopoulos, G. (2011). Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog, 7(12), e1002458-e1002458. More by Dr. George Dimopoulous: Cirimotich, C. M., Dong, Y., Clayton, A. M., Sandiford, S. L., Souza-Neto, J. A., Mulenga, M., & Dimopoulos, G. (2011). Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science,332(6031), 855-858. Ramirez, J. L., Short, S. M., Bahia, A. C., Saraiva, R. G., Dong, Y., Kang, S., ... & Dimopoulos, G. (2014). Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. About the author: Brian Lovett is a PhD student studying mycology and genetics in agricultural and vector biology systems. He is currently working on projects analyzing mycorrhizal interactions in agricultural systems, the transcriptomics of malaria vector mosquitoes, and the genomes of entomopathogenic fungi. Brian Lovett (PhD) received first place in the Student Speaking Competition for the President's Prize for his presentation entitled “Transgenic Entomopathogenic Fungi In the Semi-Field” at the Entomological Society of America Annual Meeting in Minneapolis, Minnesota held on 16 November 2015.
 Dr. Mike Raupp have been selected to receive The L. O. Howard Award from the Eastern Branch (EB) of the Entomological Society of America (ESA). This highly prestigious award was established by the EB of the ESA in 1974 to recognize scientists who have made significant contributions in the field of entomology. Dr. Raupp will be recognized by the presentation of a plaque at the annual meeting of the Eastern Branch of the Entomological Society of America taking place January 3-7, 2016 at the Society Hill Sheraton in Philadelphia, PA.  Dr. William Lamp has been awarded The Herb T. Streu Meritorious Service Award from the EB ESA. This award is to provide timely recognition to Eastern Branch members who have provided outstanding service to the society. Dr. Lamp will be recognized by the presentation of a plaque at the annual meeting of the Eastern Branch of the Entomological Society of America taking place January 3-7, 2016 at the Society Hill Sheraton in Philadelphia, PA. The Taxonomist, the Conservationist, the Agriculturalist, and the Historian In 1949, the noted nephrologist Thomas Addis described the researcher as, “Part craftsman, part practical scientist, and part historian.” Although Addis did not have parasitoid wasps in mind, this passage perfectly describes the work of Dr. Robert Kula at the USDA ARS Systematic Entomology Laboratory. Kula uses traditional insect taxonomy to answer broad scale questions about biodiversity, community ecology, and biological control of pest species. He implements large scale biodiversity studies on four continents using specimens and files from the Smithsonian Institution National Museum of Natural History (NMNH), long term fieldwork around the US, and primary literature that ranges from antiquated manuscripts to freshly minted publications. Modern scientists try to be collaborative, globally engaged researchers in order to remain competitive for positions and funding, and Kula is all of these things as a truly multidisciplinary entomologist.  Figure 1. Aleiodes indiscretus wasp parasitizing a gypsy moth caterpillar. USDA photo by Scott Bauer. Image Number K7659-1 Figure 1. Aleiodes indiscretus wasp parasitizing a gypsy moth caterpillar. USDA photo by Scott Bauer. Image Number K7659-1 Kula’s research centers on Braconidae, a hymenopteran family of parasitoid wasps. There are 19,439 described species, with ~50,000 species anticipated worldwide. Braconids range in size from 1 mm to 3 cm in length and are character rich, meaning they demonstrate a wide variety in color, sculpture, and shape (Figure 1). Like their appearance, braconid behavior is also diverse. Collectively, braconids parasitize insects from 7 orders and can either live alone (solitary) or in groups (gregarious). Some braconids permanently halt th development of their host when they lay their eggs (idiobionts, “living apart”), and others allow hosts to develop with the parasitoid offspring (koinobionts, “living together”) (Brodeur & Boivin, 2004). This braconid hyperdiversity makes the family a prime subject for studies on environmental perturbation and landscape level changes. In 2001, Kula began a large-scale sampling of braconids at Konza Prairie in Kansas. Results from previous sampling had identified only 86 braconid species, which seemed too low for a family as species-rich as the braconids. To remedy this undersampling, from 2001-2006 Kula and his colleagues collected at sites that represented a variety of land management practices (grazing, no grazing, controlled burning, etc.) (Kula & Marsh, 2011). Kula and his research group have found that braconid diversity is reflective of management strategy, with most similarity occurring between sites managed in similar ways. Thus, adjacent sites managed very differently had different profiles of braconids. Based on these initial results, Kula estimates a whopping 234 braconid wasp species will ultimately be sampled at Konza Prairie, including many undescribed species. The results demonstrate the importance of considering how grassland management affects insects that provide services critical to ecosystem function, such as parasitoid wasps. Questions that must be addressed more frequently in conservation and restoration include the following: How do we maximize biodiversity in highly fragmented ecosystems? Can small parcels of land targeted for conservation or restoration provide sufficient resources to support organisms? To what extent do parasitic wasps such as braconids move from natural habitats into agricultural fields? Kula argues that such questions are best addressed through multidisciplinary collaboration conducted in conjunction with biodiversity research programs.  Figure 2: American chestnut pre-blight. Photo: masschestnut.org Figure 2: American chestnut pre-blight. Photo: masschestnut.org More recently, Dr. Kula has embarked on a new line of research that he referred to as one of the most enthralling of his career. It benefits the American chestnut (Castanea dentata), which was formerly one of the commonest and most economically important tree species in the eastern U.S. (Fig. 2). At the turn of the nineteenth century, a devastating fungal disease referred to as ‘chestnut blight’ was transmitted to these mighty giants from introduced Asian chestnuts trees. Sadly, by 1930, American chestnut trees were ecologically extinct (Hepting, 1974). However, since the late 1980s, researchers have actively pursued a backcross breeding program that confers the Chinese chestnut tree’s blight resistance to its American cousin, resulting in a hybrid that is 94% American and 6% Chinese (Herbard, 2006) With chestnut tree revival on the horizon, Dr. Kula and his collaborators began to ask several questions, including “how did the functional extinction of the American chestnut impact its community of associated insects”, and “do herbivores on hybrid chestnut experience enemy-free space (release of pressure from natural enemies) from parasitic wasps”. This led Kula and his collaborators to develop a project on American chestnut and its associated insect fauna both pre- and post-blight. Uncovering pre-blight fauna is done by consulting literature from that period, as well as historical insect records in the Hopkins Notes and Record System, maintained in the Department of Entomology at the NMNH. This extensive recording system used by the USDA from 1899 until the 1980s contains detailed notes about insects of U.S. forests, including herbivores on American chestnut and their natural enemies. A comprehensive literature review indicated that most reports of insects associated with endemic or exotic chestnut in the U.S. are from five species: two putatively extinct micromoths, gypsy moth, chestnut gall wasp, and small chestnut weevil. Other researchers have considered four moth species extinct due to loss of America chestnut from the forest canopy. Kula and his collaborators are assessing pre- and post-blight trophic associations to understand herbivore-natural enemy interactions in the context of chestnut restoration  Figure 3: Tree canopy trap. Photo credit: Robert Kula Figure 3: Tree canopy trap. Photo credit: Robert Kula The first step in assessing the impact of American chestnut loss and recovery on associated herbivores and natural enemies is determining the extent to which insects historically associated with American chestnut occur in its ancestral range. Dr. Kula and his group, consisting mostly of Smithsonian Institution interns, set up insect flight traps (Fig. 3) in the canopies of pure American chestnut, Chinese chestnut and northern red oak trees. Traps were placed in Chinese chestnut and red oak trees because they are in the same plant genus and family, respectively, and red oak co-occurred with American chestnut throughout its historical range. One of his most exciting finds so far is a parasitic wasp reported previously from one of the two micromoths presumed extinct. This finding opens up further avenues of research: are there still viable populations of the moth species, or was the wasp species able to shift hosts? Is the host range for the wasp broader than what was reported initially? Hand-collecting herbivores on American chestnut and related tree species will no doubt shed more light on herbivore-natural enemy interactions from contemporary and historical perspectives. Dr. Kula’s research nicely demonstrates how taxonomy and natural history collections can be used to simultaneously address an array of questions in different, yet interdependent, areas of biology. References: Addis, Thomas, Helmuth Bookplate: Sprinz, and c1948 1949. Glomerular Nephritis: Diagnosis and Treatment. New York: Macmillan, 1949. Brodeur, J., & Boivin, G. (2004). Functional ecology of immature parasitoids. Annual Reviews in Entomology, 49(1), 27-49. Hebard, Fred V. "The backcross breeding program of the American chestnut foundation." Journal of the American Chestnut Foundation 19 (2006): 55-77. Hepting, George H. "Death of the American chestnut." Journal of Forest History 18.3 (1974): 61-67. Kula, R. R., & Marsh, P. M. (2011). Doryctinae (Hymenoptera: Braconidae) of Konza Prairie excluding species of Heterospilus Haliday. Proceedings of the Entomological Society of Washington, 113(4), 451-491. About the authors:
Lisa Kuder is a PhD student in Dennis vanEngelsdorp’s lab. Broadly, her research focuses on pollinator habitat along highway rights-of-way. More specifically, she is looking at the effects of de-icing salt on the floral resources of roadside wildflowers and ramifications for foraging insects. Gussie Maccracken is a PhD student in Dr. Charlie Mitter’s Lab. Her research focuses on deep-time plant-insect associations. She is currently studying 75 million year old insect damaged leaves from western North America.  Photo Courtesy of Sam Droege Photo Courtesy of Sam Droege Olivia Bernauer’s research in Dennis vanEngelsdorp’s Bee Lab was featured in the Tuesday, December 8 edition of the Diamondback. Click below to read the article which covers her efforts to expand our understanding of the flower preferences of the tremendous diversity of bees found through the state. Congratulations on your feature Olivia! University of Maryland Student Studies Flower Preferences of Bees Phylogenetics and Bioinformatics Pair to Unravel Trichopteran Evolutionary Mysteries  Fig. 1: A drawer from the Smithsonian Institution’s Trichoptera collection (Frandsen 2015) Fig. 1: A drawer from the Smithsonian Institution’s Trichoptera collection (Frandsen 2015) Trichoptera, or caddisflies, comprise the most diverse strictly aquatic insect order. The desire to understand the cryptic evolutionary background of such incredible diversity has inspired an eager phylogeneticist, Dr. Paul Frandsen, to research Trichoptera. As Dr. Frandsen began his talk about Trichoptera, it became clear that he was fascinated by their wide-ranging behaviors and appearances (Fig. 1). Listening to him talk, I began to share his fascination. Trichoptera has three suborders: Integripalpia, Annulipalpia, and Spicipalpia. Integripalpia create mobile homes out of passing junk such as twigs, rocks, and tubes. Some Integripalpia even develop a fondness for bling and make a home out of jewels.  Fig. 2: Trichoptera larvae making jewelry (Matus 2012) Fig. 2: Trichoptera larvae making jewelry (Matus 2012) These caddisfly larvae are great jewelry-makers (Fig. 2)! Annulipalpia also known as the “fixed-retreat makers” are unique for their ability to spin elaborate capture nets, filters, and funnels out of silk. Dr. Frandsen described some of their living quarters as appearing “like twigs sticking up in sandy soil”. Larvae of Spicipalpia range from making cases to being free-living, but most make cases as pupae. In terms of feeding, some Trichoptera are filter-feeders while others are predators. Dr. Frandsen is particularly interested in trying to discern the evolutionary divergence of families of Spicipalpia, a suborder that is still taxonomically unclear. The families of Spicipalpia are Rhyacophilidae, Hydrobiosidae, Glossosomatidae, Hydroptilidae, and Ptilocolepidae. 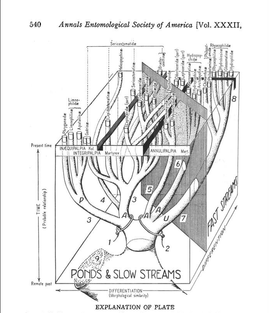 Fig. 3: Diagram showing the evolutionary differentiation of the suborders of Trichoptera from ponds to faster streams (Milne and Milne 1939) Fig. 3: Diagram showing the evolutionary differentiation of the suborders of Trichoptera from ponds to faster streams (Milne and Milne 1939) The process of resolving the “story” of how these families evolved currently draws from disciplines and techniques more aligned with the cloud computing technology of tech giants than traditional scrutiny of insect samples from museums. However, the first record of attempting to understand Trichoptera evolution appears in a paper by Milne and Milne in 1939, in which the authors proposed an evolutionary history where Trichoptera moved from ponds to slow moving streams to fast moving streams (Fig. 3). The authors of that paper considered two situations where Trichoptera anatomy “fit” the story. In their hypothesis the Rhyacophilidae are considered a more recently diverged family of Trichoptera. Currently, entomologists still have not completely resolved the evolutionary history of Trichoptera, but alternative evolutionary trajectories that suggest Rhyacophilidae are actually more ancestral have been supported by recent studies (Malm et al. 2013). 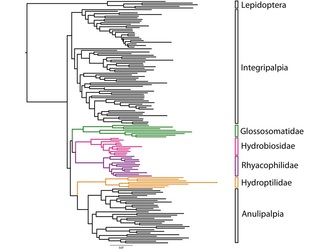 Fig. 4: Phylogenetic tree of Trichoptera families created using the k-means algorithm for individual DNA patterns in genes (Frandsen et al. 2015). Fig. 4: Phylogenetic tree of Trichoptera families created using the k-means algorithm for individual DNA patterns in genes (Frandsen et al. 2015). In an effort to resolve disagreements about how these organisms are related to each other, Dr. Frandsen has decided to tackle the problem using modern biotechnological techniques. DNA was sequenced en mass among many species. The data that resulted from this mass genetic analysis of these organisms was enormous. When different analysis and modeling techniques were used to map the relationships between Trichoptera, a puzzling inconsistency was found between data sets. Each technique seemed to be telling a different story that did not match what was previously known about Trichoptera biology and appearance. Dr. Frandsen then decided to collect even more samples, this time using DNA protein-coding regions called exons. This technique is unique as it only selects areas that are part of the final mRNA (after editing) used to transcribe proteins. This again yielded another enormous dataset that required super-computing effort to decipher. In order to do this, Dr. Frandsen developed a new model selection algorithm (Frandsen et al. 2015). Essentially, he co-opted the k-means clustering algorithm used by technology companies to group “people” or groups of people by the trends those people are following. For Dr. Frandsen, the “trend” computed would be Trichopteran genes and instead of people, individual DNA patterns. For example, rather than grouping people who visit shoe websites together, the mathematical algorithm was used to determine which DNA sites have the most similar evolutionary patterns and thus should be modeled together. After analyzing the information generated from the algorithm, a phylogenetic tree was made to show the evolutionary relationships of Trichoptera taxa (Fig. 4). Dr. Frandsen was then able to address some of the ambiguity of the Spicipalpia families. Dr. Fransen’s phylogenetic tree (Figure 4) shows that Glossomatidae are a basal family. Hydrobiosidae and Rhyacophiladae diverged later. The Hydroptilidae diverged separately as an independent clade, suggesting that the Spicipalpia are polyphyletic. Currently, Dr. Frandsen is working on analyzing the evolutionary relationships between Trichopteran transcriptomes (the set of all RNA molecules transcribed in a cell or population of cells), and how Ptilocolepidae, an enigmatic Spicipalpian family, can be incorporated into the phylogenetic tree (Frandsen 2015). Results from this analysis provide a new picture of how caddisfly families relate to each other and reveals how big data may help answer questions on other cryptic species and overall evolutionary history of life on planet earth. Despite his extensive undertaking, Dr. Frandsen believes that there is still much more work that needs to be done. There is ongoing research to discover better ways to create models, develop algorithms that are faster and more accurate, and create hardware that can process much more data. The field of bioinformatics is exciting and still quite young, so the potential for entomological applications seems very promising. References: Frandsen et al. (2015), Automatic selection of partitioning schemes for phylogenetic analyses using iterative k-means clustering of site rates. BMC Evolutionary Biology, 15:13 Frandsen, Paul (2015), Phylogenetic model selection and data filtering on large data sets: a case study with the caddisflies (Insecta: Trichoptera). University of Maryland Colloquium. Malm, T., Johanson, K. A. and Wahlberg, N. (2013), The evolutionary history of Trichoptera (Insecta): A case of successful adaptation to life in freshwater. Systematic Entomology, 38: 459–473. doi: 10.1111/syen.12016 Matus, Morgana (2012), Artists Enlist Caddisfly Larvae to “Build” Jewelry From Gold, Gemstones. Ecouterre Milne, J. and M.J. Milne (1939), Evolutionary trends in caddis worm case construction. Annals of the Entomological Society of America, 32(3): 540, Fig. 1 About the Authors:
Hanna Kahl and Armando Rosario-Lebronare are graduate students at the University of Maryland in the Entomology Department. Rahul Raiker is an undergraduate student at the University of Maryland in the Molecular Biology Department. |
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290

 RSS Feed
RSS Feed




