|
It can be a lonely world for a bed bug researcher! Dr. Mark Feldlaufer began his presentation by extending an open invitation to visit his bed bug research lab at the United States Department of Agriculture-Agricultural Research Center in Beltsville, Maryland. He assured us that the offer rarely gets takers, and it is really not surprising, all things considered. As parasites that feed on people and cause itchy welts, bed bugs give people the heebie-jeebies. Just a picture or two of the bed bugs (Fig. 1) was enough to have several attendees visibly cringe, as they imagine the marks and persistent itches that usually follow after bed bug bites. However, Dr. Feldlaufer’s fascinating research, which aims to improve bed bug detection and control, may contribute to a future where everyone can rest assured that there truly are no creatures lurking under the bed.
often do not come in contact with them. It is impractical, dangerous, and against labelled usage, to coat every surface and crack of a home or business in insecticide, so bed bug chemical treatments often fail. The silver bullet control method appears to be heat treatments, but, like silver, heat treatments are expensive. Homes are heated to between 120-140⁰F for several hours to kill all bed bug life stages, which tend to cost anywhere between $2,500-$7,000. Though bed bugs themselves do not discriminate against the rich or poor, our methods of remediation tend to do just that. The most effective way to get rid of bed bugs is unaffordable to many.
In the course of his bed bug research, Dr. Feldlaufer has worked on other projects to help to detect and control bed bugs early in their infestations with a number of students, including our very own Dr. Kevin Ulrich. The team found that bed bugs consistently avoided a common chemical insect repellent, DEET, the main ingredient in most mosquito repellents. They did not, however, respond with greater avoidance to higher dosage. Although this may seem like a quick and easy fix, wearing DEET to bed may just cause bed bugs to merely move on to other surfaces (e.g. the couch). On the other hand, they also found that aldehyde compounds produced by bed bugs elicit a strong attractive response to other adult and immature bed bugs. These aldehydes may be one way bed bugs attract each other to form aggregations (Ulrich et al. 2016), suggesting that the aldehydes may be used to develop an inexpensive and more discreet option to lure and trap bed bugs. Dr. Feldlaufer currently aims to develop and test reduced risk pesticides that show promise in killing bed bugs. Bed bugs can be a terribly menacing presence in anyone’s home, but thankfully Dr. Feldlaufer has dedicated much of his career to keeping us informed about these insects and their crafty activities. By pairing lures with reduced risk pesticides, Dr. Feldlaufer aims to develop options to safely, affordably, and discreetly ensure we can sleep tight assured that the bed bugs will not bite. References: Lit L., Schweitzer J.B., and Oberbauer A.M. 2011. Handler beliefs affect scent detection dog outcomes. Animal Cognition 14: 387. Pfiester M., Koehler P.G., and Pereira R.M. 2008. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. Journal of Economic Entomology 101(4):1389-96. Ulrich, K.R., Kramer, M. and Feldlaufer, M.F. 2016. Ability of bed bug (Hemiptera: Cimicidae) defensive secretions (E)-2-hexenal and (E)-2-octenal to attract adults of the common bed bug Cimex lectularius. Physiological Entomology 41: 103-110. Author Bio: Hanna Kahl is a master’s student at University of Maryland in Dr. Cerruti Hooks’ lab researching the effects of red clover living mulch on arthropod pests and pollinators. Samuel Ramsey is a PhD student at University of Maryland in Dr. Dennis vanEngelsdorp’s lab researching Varroa destructor. Skunk, cilantro, burnt rubber—just a few of the many scents you might associate with the pungent odor produced by stink bugs. Like many animals, these bugs produce a variety of chemical odors (called semiochemicals) that modify the behavior of recipient organisms in different ways. That “stink” that these bugs get their name from is just one example. Stink bugs (family Pentatomidae) represent an extremely diverse family of insects that includes both agricultural pests and beneficial predators. Some of the agricultural pest species can cause millions of dollars in damages to crops. At the USDA Agricultural Research Service in Beltsville, Maryland, Dr. Don Weber studies stink bug semiochemicals in hopes of being able to take advantage of their communication to monitor their populations. In particular, Dr. Weber studies pheromones – one kind of semiochemical used for communication within a species. For example, male stink bugs emit pheromones to attract females, or in other cases, both females and males. In other insects, pheromones can also act to signal danger, food resources, or aggregation sites. Because semiochemical signals often influence insect behavior, synthetically replicating these scents equips growers with a powerful tool to manage agricultural insect pests. This strategy works most effectively if the chemical composition of the natural odor is identified and isolated, and if the behavior it elicits is fully understood. The invasive brown marmorated stink bug (BMSB) (Halyomorpha halys) and the harlequin bug (Murgantia histrionica) (Figure 1) both respond to traps baited with pheromone lures which can be used in order to monitor levels of these pests to determine whether or not to take action against them with insecticides (Figure 2). With stink bugs, there are some challenges that researchers such as Dr. Weber have faced in using pheromones as lures. Pheromones released by male stink bugs are not exclusively sex pheromones;females, males and nymphs alike are attracted by the male pheromones. This indicates to researchers that those pheromones might have some of the other roles mentioned above, such as signaling aggregation sites. Yet another piece to this complicated smell puzzle stems from cross-species attraction, meaning that the deployment of a pheromone by one stink bug species can attract another stink bug species. Scientists can monitor BMSB by using MDT, the aggregation pheromone of a completely different stink bug species (the Asian brown-winged green bug, Plautia stali). However, because the pheromone is most attractive to BMSB in the fall, which is after most crop harvests have finished or already starting, so this is not very helpful for most growers1. To produce better attractants for BMSB, researchers successfully identified the specific aggregation pheromone produced by BMSB adult males. The pheromone (commonly called murgantiol) is comprised of two different isomers, or molecular conformations, with a specific ratio. This ratio is important to know because there can be variation between species, and even between individual bugs, for what ratio is most attractive. Dr. Weber and colleagues determined which isomer ratio of murgantiol was most attractive separately to BMSB, and then combined this pheromone with MDT to see if a blend of the two was attractive to the bugs. The two pheromones together were much more attractive than either alone – with an added advantage of this blend being attractive to the bugs all season long, unlike MDT by itself. These pheromones also do not need to be extremely pure, which is good news for keeping the cost of lure production low. Harlequin bug males produce murgantiol as well – so the same types of lures can be used to monitor both pest species, though the two species have different ideal isomer ratios5. Harlequin bugs, however, specialize in feeding on cruciferous plants – unlike BMSB, which are unspecific in their preferred hosts (polyphagous). Harlequin bugs were found by Dr. Weber to also be highly attracted to pheromones mixed with mustard oils, which are chemically derived from their host plants’ defensive compounds6,7. Adding these oils to murgantiol therefore could enhance trap performance for harlequin bugs, much like MDT for BMSB. While Dr. Weber and his colleagues uncovered a lot of information about stink bug pheromones, there is still room to further our understanding. Now that researchers have evidence for which pheromone blends work most effectively for the two species, there is a lot of fine-tuning to be done about how exactly to use them in traps, and the most economical way to produce them. Dr. Weber and his colleagues also have plans in the works to investigate using pheromones for other pest-combating purposes, such as attracting insect predators of pest species like stink bugs, and genetically engineering plants to produce insect pheromones and act as trap crops. Overall pheromones offer an exciting approach to manage not only stink bugs, but many different pest insects, to better protect our agriculture. To learn more about Dr. Weber’s research or contact him, visit his USDA homepage. For more information on BMSB or Harlequin bugs, you can visit the University of Maryland Extension pages on BMSB and Harlequin bugs. Authors: Elizabeth Brandt, Aditi Dubey, Morgan Thompson Resources Cited
However, asymptomatic bees are common and can have either low or high virus loads (de Miranda et al. 2012). The story is complicated by the fact that DWV is very closely related to a series of other RNA viruses, such as Varroa destructor virus-1 (VDV-1). And the story gets even more complicated… because of recombination.
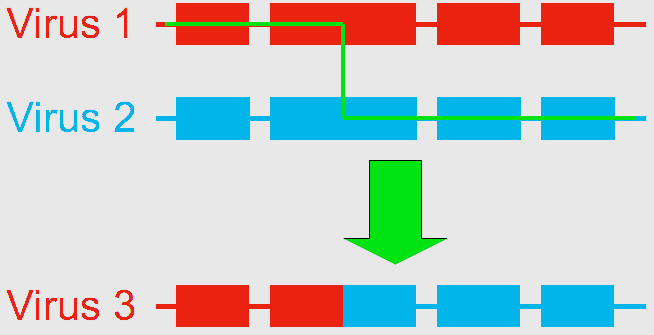
Using next generation gene sequencing, Dr. Ryabov (currently a visiting scientist at the United States Department of Agriculture (USDA) Bee Research Lab in Beltsville, MD) and his colleagues at the University of Warwick, UK, decided to characterize the virus diversity in honey bees. They found that the genome of DWV-like viruses could be divided into three functional parts, or “modules”, any of which were sometimes crossed-over between DWV and VDV. They identified three distinct types of genomes: the 100% DWV genome, and two types of recombinants formed by the association of “modules” from DWV and VDV, which they named VDV-1DVD and VDV-1VVD (Moore et al. 2011). So what was thought to be a single population of viruses is actually a group of variants (VDV-1DVD, VDV-1VVD and DWV). Dr. Ryabov then compared the levels of each variant in honey bee pupae and associated Varroa mites. They found that individual honey bees were usually infected by a mixture of the three variants. Of the three viruses, the recombinant VDV-1DVD’s levels in honey bee pupae was highly associated with its level in associated mites. This suggests this recombinant is more efficiently transmitted between the mite and the honey bee. When Varroa acts as a vector for the DWV, it increases the levels of viruses in contaminated colonies, causes deformities in the affected workers (Fig. 2), and overall results in increased risk of mortality of the whole colony. But what remains to be determined is whether this is caused by 1) Varroa amplifying and introducing more virulent strains of the virus and/or by 2) Varroa suppressing the honey bee immune response. To test those two hypotheses, Dr. Ryabov exposed Varroa-naïve honey bees (collected from a Varroa-free region in Scotland) to DWV either orally (in brood food) or through Varroa mite feeding. They monitored the change in DWV diversity and loads within the host as well as changes in honey bee expressed genes to identify potential antivirus immune responses (Ryabov et al. 2014). They detected changes in expression for a number of genes associated with the immune response of honey bees while in presence of the mites. This suggests that the second hypothesis should be further explored. This study also showed that in Varroa-free colonies (controls), honey bees had highly diverse DWV, though at low levels. When the bees were infected orally, DWV levels remained low, but the composition of the DWV strains changed compared to the controls. When bees were infected through Varroa, two outcomes would happen. Honey bees either showed low levels of diverse DWV strains, or they developed high levels of a single specific variant of DWV or very closely related variants, even though the infecting mite contained a high diversity of strains. By inoculating honey bees through injections (which simulates Varroa feeding), the researchers observed high levels of replication for the recombinant strains containing VDV-1 derived structural gene block. This suggests that these particular strains have an advantage due to the route of transmission. All of this largely supports the first hypothesis that Varroa amplifies more virulent strains of the virus. In conclusion, this example of the shift in virulence of the DWV – from a benign and asymptomatic virus to a serious disease – illustrates the importance of the process of recombination in the generation of various strains of viruses, and how the addition of a vector, and a new route of transmission, can increase the impact of a virus by altering the relative composition of its strains. Bloggers: Meghan McConnell is a Master’s student in Dennis vanEngelsdorp’s Lab. She is currently studying honey bees, with a focus on non-chemical control of Varroa mites. Nathalie Steinhauer is a PhD candidate working in Dennis vanEngelsdorp’s Lab on honey bee health and management practices. Her projects aims to identify and quantify the effects of risk factors associated with increased colony mortality. References: Bowen-Walker, P. L., S. J. Martin, & A. Gunn. 1999. The Transmission of Deformed Wing Virus between Honeybees (Apis mellifera L.) by the Ectoparasitic Mite Varroa jacobsoni Oud. Journal of invertebrate pathology 73(1), 101-106. Highfield, A. C., A., El Nagar, L. C. Mackinde, M. L. N. Laure, M. J. Hall, S. J. Martin, & D. C. Schroeder. 2009. Deformed wing virus implicated in overwintering honeybee colony losses. Applied and environmental microbiology 75(22), 7212-7220. de Miranda, J. R., L. Gauthier, M. Ribiere, and Y. P. Chen. 2012. Honey bee viruses and their effect on bee and colony health. In D. Sammataro & J. Yoder (Eds.) Honey bee colony health: challenges and sustainable solutions. CRC Press. Boca Raton. 71-102. Moore, J; Jironkin, A; Chandler, D; Burroughs, N; Evans, DJ; Ryabov, EV (2011) Recombinants between Deformed wing virus and Varroa destructor virus-1 may prevail in Varroa destructor-infested honeybee colonies. Journal of General Virology, 92(1): 156–161. DOI:10.1099/vir.0.025965-0 Ryabov, EV; Wood, GR; Fannon, JM; Moore, JD; Bull, JC; Chandler, D; Mead, A; Burroughs, N; Evans, DJ (2014) A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis mellifera) Prevails after Varroa destructor-Mediated, or In Vitro, Transmission. PLoS Pathogens, 10(6): 1–21. DOI:10.1371/journal.ppat.1004230 Aliens are invading the forests of the United States! Not the green, bug-eyed aliens from outer space; no we are talking about the, well… green, bug-eyed aliens from Earth. With the globalization of trade, insect introductions leading to invasive pest problems have steadily increased over the last few centuries, causing massive economic and environmental devastation in the systems where these pests permeate. These invaders are especially difficult to manage when they are pests of our native North American forest trees due to the large spatial scale associated with them, making pesticide applications impractical.
having a warmer climate than Connecticut, created an asynchronous relationship between the host (EHS) and the parasitoid (E. citrina) in Connecticut. This means that the scale and parasitoid are developing at different times of year, preventing the wasp from being able to effectively attack the scale in its introduced range. With the colder climate of Connecticut, it was hypothesized that the EHS scales developed more slowly. Wasps, as a result, would have fewer suitable 2nd instar hosts to parasitize. Dr. Abell tested this by observing scale abundance and parasitism by E. citrina at three distinct latitudes in the U.S. (Connecticut [“coldest”], Pennsylvania, North Carolina [“warmest”]), hypothesizing that he would find more parasitoid-host synchrony as he moved further south where warmer temperatures would allow for multiple generations of scales. Ultimately, Dr. Abell did not observe any increase in synchrony between EHS and E. citrina at any of his three field sites. Instead he found continuous reproduction of EHS, and all life stages were present throughout the year. This led Dr. Abell to Japan to better understand how EHS behaves in its native range. While surveying hemlock scales and their associated parasitoids, Dr. Abell found 11 new species attacking EHS in Japan, some of which may have potential as classical biological control agents.
a wasp that is less than 1mm in length that attacks EAB eggs. Research done by Duan et al. in 2013 indicated that T. planipennisi was effectively established in Michigan and is a strong disperser. However, they observed that there was no parasitism of EAB in larger trees. In a study done by Dr. Abell, it was determined that the bark thickness was preventing this small wasp from attacking the EAB larvae. The ovipositor (egg-laying mechanism) of T. planipennisi is too short to reach the EAB larvae underneath the thick bark.
The bark was also placed in emergence chambers to collect any parasitoid wasps that emerged from the bark remnants that were missed in earlier screening. After two years of testing these methods, Dr. Abell concluded that the bark-sifting method was a more effective way to measure the rate of O. agrili egg parasitism in the field because significantly more parasitoids were recovered with this method. Invasive insects continue to attack our forests today, therefore it is very important to continue to understand and utilize biological control methods to preserve our forests. Dr. Abell continues his work on EAB biological control in the Shrewsbury lab here at the University of Maryland where he is evaluating other introduced and native parasitoids and additionally an integrated approach that combines pesticides with classical biological control methods.
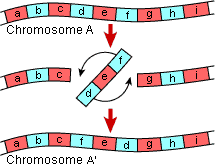
About the Authors: Olivia Bernauer is a second year Master’s student in Dennis vanEngelsdorp’s bee lab working to better understand the floral preferences of Maryland’s wild, native pollinators. Jackie Hoban is a second year Master’s student working on emerald ash borer biological control in Paula Shrewsbury’s lab. What makes a species a species? What are the characteristics that make it unique enough to be distinguished from similar creatures? What are the genetic underpinnings that allow species to evolve to create a distinct entity? These are the type of questions Dr. Carlos Machado is trying to answer. Answering them are key to understanding our planet's biodiversity. A traditional definition of speciation centers around the concept of reproductive isolation. While members of two different species may mate, if they either produce no offspring or sterile offspring, these two individuals represent different species. This is known as the biological species concept. However, what factors lead to reproductive isolation? One way is through physical separation of populations (e.g., a mountain range) or allopatric speciation. On the other hand, what about sympatric speciation, or speciation in overlapping geographical distributions?
Dr. Machado’s work brings us a step closer to understanding the roots of speciation. Following from this work, his future research ideas center on correcting inversions using the latest genetic techniques, such as CRISPR (Clustered regularly interspaced short palindromic repeats), to study in further detail these regions that are responsible for species’ differences. Jonathan Wang is a PhD student in Raymond St. Leger’s lab studying host-pathogen interactions. Jen Jones is a PhD student in Bill Lamp’s lab, studying how socioeconomic factors influence the distribution of mosquito populations. As the world’s population continues to grow, a key issue facing society is how to balance feeding the world while protecting the environment. Dr. Kate Tully has worked on this problem in both East Africa and the United States, where the solution will require different approaches, in part due to the different use of crops. In developed nations, many grain crops are grown for animal feed and fuel compared to developing nations where most are grown for human consumption. As such, different goals and approaches need to be taken for a sustainable future. While crop yield has increased in many parts of world since the 1960s, this trend has not occurred in sub-Saharan Africa. Dr. Tully is working in Kenya and Tanzania to determine what changes can improve yield without excessive fertilizer use. Africa as a whole plans to increase nitrogen-based fertilizer use, but there is an upper limit to the amount plants can use before the rest is lost to the environment.
Large amounts of fertilizer nitrate were leached through the sandy soils to groundwater. Dr. Tully concluded that, in addition to using better overall management practices such as irrigation and increasing organic matter content, site characteristics should determine the optimal nitrogen fertilizer amount; in these cases, farmers in Kenya can increase yield by adding 75-100 kg N/ha while those in Tanzania should only use 50 kg N/ha. These levels can increase yield while protecting the environment from excess nitrogen leaching into waterways and gassing off into the air. Back in the U.S., Dr. Tully turned her attention to soil nutrient dynamics on the Eastern Shore, Maryland. The dominant agricultural crops feed—corn and soybeans support their primary industry—poultry. Over the years, phosphorus from fertilizers and chicken litter has accumulated in the soil to the point of saturation. Phosphorus typically binds to soil particles with minimal nutrient leaching to the surrounding environment. Yet, as human-accelerated sea-level rise causes saltwater intrusion of coastal farmland, soil dynamics are changing. Saltwater can significantly alter soil chemistry, which may result in the release of bound soil nutrients as runoff. While nitrogen and phosphorus are essential nutrients for living organisms, too much can throw entire ecosystems off balance. Excess phosphorus in particular has led to algal blooms in fresh water estuaries. Over time algal blooms deplete life supporting oxygen resulting in ‘dead zones.’ But to what magnitude, rate, and extent are saltwater intrusions increasing the movement of phosphorus?
Read more of Dr. Tully’s work!
Tully K, Hickman J, McKenna M, Palm CA, Neill C. 2016. Effects of increased fertilizer application on inorganic soil N in East African maize systems: vertical distributions and temporal dynamics. Ecological Applications 26: 1907-1919. doi:10.1890/15-1518.1 Becca Eckert is a Ph.D. student in Bill Lamp’s lab. Her research examines how changes in light and nutrient availability affect macroinvertebrate growth and diversity in heterotrophic headwater streams as mediated by changes in leaf-associated algal quantity and quality. Lisa Kuder is a Ph.D. student in Dennis vanEngelsdorp’s Bee Lab. Her research focuses on road ecology, specifically improving highway rights-of-way for pollinators. Resurrecting the Work of Gordon Alexander: Grasshopper Communities and our Changing Climate9/23/2016
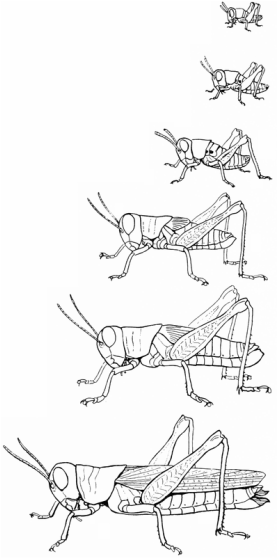
Dr. César Nufio has examined a vast number of grasshoppers to understand the impacts of climate change on insects in the Rocky Mountains of Colorado. To be precise, the number of specimens he has captured and processed over the last 10 years has exceeded 180,000 from grassland communities found along a high plains to subalpine gradient. Nufio’s National Science Foundation funded research combines extensive field surveys with comparisons of museum collections, weather data, and laboratory and field experiments. The entire project started with his discovery of a collection of 25,000 pinned and label grasshopper specimens and three data notebooks at the University of Colorado’s Natural History Museum. These pinned specimens and notebooks, which are part of the Gordon Alexander Collection, were part of several field studies conducted over 50 years ago.
In 2006, Nufio began resurveying the same sites that Alexander had nearly half a century ago. Nufio wanted to understand how climate had changed along the gradient and how this change might impact the timing of grasshopper life history events (when they hatched and how fast they developed), their elevational ranges and demography (population size, longevity, reproductive rates), and body sizes. All of these questions could be addressed comparing his recent findings to the observations made by Alexander.
In addition to examining phenology, Nufio more recently was interested in the demographic changes among species over the elevational gradient. He observed that species with short wings showed a reduction in body length with no change in reproductive output with increasing elevation. Conversely, grasshoppers with long wings showed no change in body size but a reduction in reproductive output. Nufio also examined changes in weight, longevity, and reproduction over time in response to temperature using a caged field experiment. During a warm year, he found that females tended to be heavier, live longer, and laid more eggs (more on demography: Article). While these subalpine grasshoppers appear to be benefiting from warming, his surveys suggest that those at the bottom of the mountain may be negatively affected by warm years.
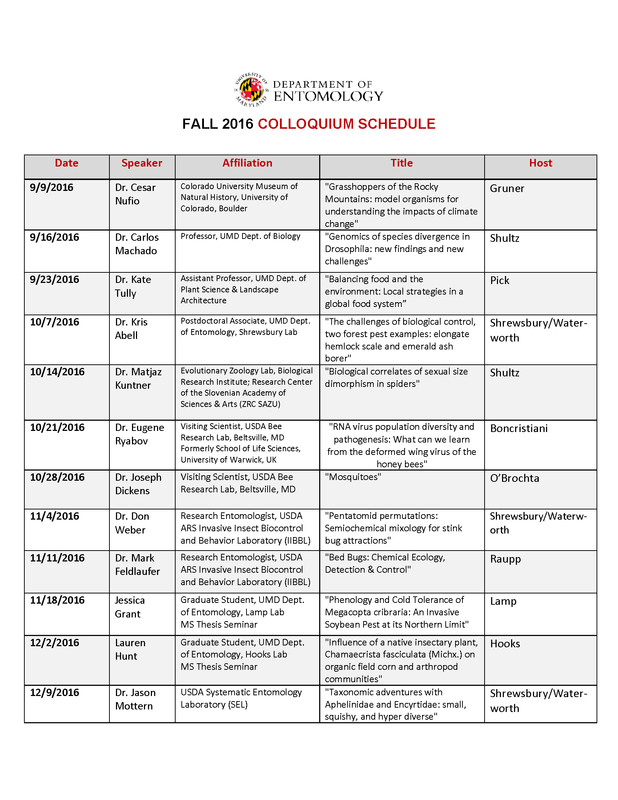
Please join us for as many as you can! Lunch will be provided for all attendees.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290



















 RSS Feed
RSS Feed




