|
What drives species apart: how does evolution turn one population into two populations that can no longer mate? This process is called speciation, and we have a lot to learn when it comes to this subject. Dr. Catherine Linnen, from the University of Kentucky, is using pine sawflies (genus Neodiprion) as a model to better understand the origins of biodiversity, from its proposed start as genetic mutations to eventual speciation. Pine sawflies have particularly diverse characteristics (or phenotypes) in terms of their body shape, larval coloration, social behavior, and host preference, though most use some type of pine trees. Since some of these species are pests, much their biology has been well studied. Speciation is easier to study in organisms showing divergent behaviors, preferences, and morphologies because these are all characteristics that evolution is proposed to act upon in the process of speciation. These qualities, in addition to the ease of their capture and lab-rearing, make them perfect for studies on speciation. How are differences in phenotype, such as host selection, driving populations apart into distinct species? What specific mutations are responsible for variations in phenotypes? Dr. Linnen proposed a phenotypic approach, which can be used to identify underlying genetic variation, using Neodiprion populations to help answer these questions 1. Can host preference separate populations into distinct species? In theory, this could happen in three steps: first, the colonization of a novel host; second, divergent selection on different traits; third, a reduction in gene flow leading to speciation. With a sizeable amount of biological data, Dr. Linnen was able to show that host use was the best predictor to model the speciation seen in Neodiprion species. Dr. Linnen showed an interesting example of this from her work on the saw-shaped egg-laying apparatus of adult sawflies, which is where these organisms get their common name. Different sawfly species have saws and eggs that fit well into the needles of specific host plants. In this case, she was investigating saw and egg differences of sawflies on pine trees with thick or thin needles. Since individuals with a thick saw would have lower fitness on host plants with thin needles, and since thin-sawed sawflies would ill-equipped to exploit hosts with thick needles, this trait could drive speciation. When looking at mitochondrial genes for proof of introgression (exchange of genes between distinct populations as a result of mating) between species, she showed that host difference seems to act as a barrier for introgression, as well. 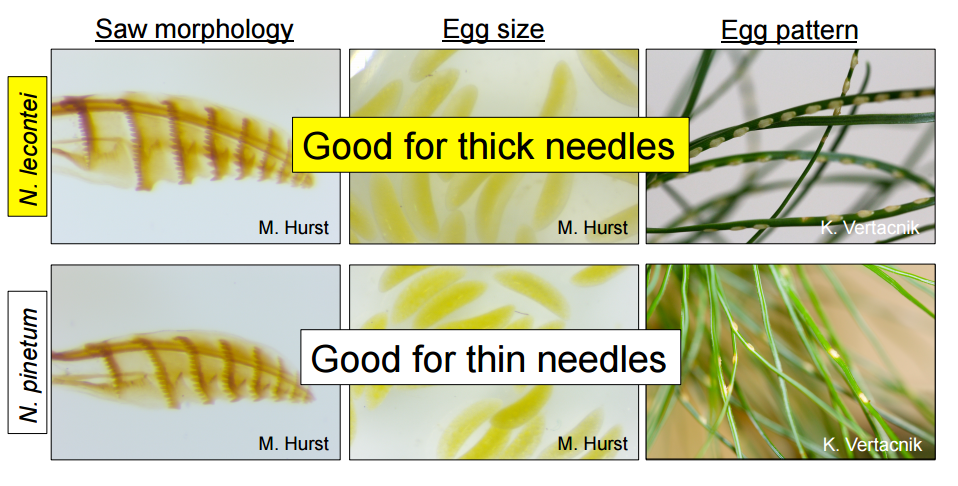 Image shows variation in saw size and shape, egg size, and the typical spacing of eggs laid on different pine tree needles by Neodiprion lecontei and Neodiprion pinetum. These differences allow for these two species to specialize on different species of plants leading to potential spatial isolation even within the same geographic range. 2. Is there evidence of the same type of processes at the population level?
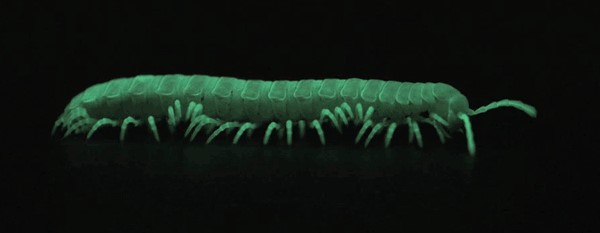
If yes, two individuals from the same species, but with different hosts should show higher genetic variation than a pair from the same host. The observed data supported this trend. After accounting for genetic variation due to geographical clusters, Dr. Linnen showed that divergent selection of hosts could drive differentiation between populations of a single Neodiprion species, potentially leading to speciation. 3. But how? Is host use reducing inter-breeding or is there a cost associated with inter-breeding in terms of fitness? Neodiprion females show preference when egg-laying: each prefer to lay eggs on their typical host. This behavior alone explains some spatial isolation resulting in reduction in gene flow. In addition, when females and males are placed together to test for mating preference, they prefer to mate with their conspecific (an individual of the same species), even in the absence of any host. This behavior indicates some level of sexual isolation, which would also reduce gene flow. However, in the lab, some individuals still chose a non-conspecific mate from the other host, resulting in hybrids. Hybrids were also collected in the field. Those observations, plus the results showing some level of mitochondrial introgression suggests that sexual isolation between Neodiprion species is incomplete. Host preference is associated with both survival and fecundity. Each species survives and reproduces better on its typical host. This amounts to a fitness cost for hybrid phenotypes resulting in underperformance compared to specialists on either host. Egg mortality was strongly linked to the matching of oviposition traits to the hosts. In particular, traits associated with thick-needles resulted in high mortality rates when eggs were laid on pines with thinner needles. What can sawflies tell us about speciation? Dr. Linnen’s work suggests that pine needle width is generating divergent selection pressure on host traits within and between populations of sawflies resulting in fitness costs for hybrid individuals and eventually driving speciation. By just considering how they lay eggs, sawflies can tell us a great deal. There is still more to learn as we explore the diversity of sawfly traits. References: Linnen, C.R. and B.D. Farrell. 2010. A test of the sympatric host race formation hypothesis in Neodiprion (Hymenoptera:Diprionidae). Proceedings of the Royal Society of London B. 277: 3131-3138. Bagley, R.K., V.C. Sousa, M.L. Niemiller, and C.R. Linnen. In Revision. History, geography, and host use shape genome-wide patterns of genetic differentiation in the redheaded pine sawfly (Neodiprion lecontei). Bendall, E.E., Vertacnik, K., and C.R. Linnen. In Prep. Selection on oviposition traits generates reproductive isolation between two pine sawfly species. About the Authors: Nathalie Steinhauer is a PhD candidate working in Dennis vanEngelsdorp’s lab on honey bee health and management practices. Her projects aims to identify and quantify the effects of risk factors associated with increased colony mortality. Samuel Ramsey is a doctoral student in Dennis vanEngelsdorp’s lab. His work focuses on honey bee parasites specifically targeting ectoparasite Varroa destructor and endoparasite Nosema ceranae. His project seeks to establish a deeper understanding of key aspects of both parasites’ biology with the aim of optimizing current IPM practices in respect to these organisms. 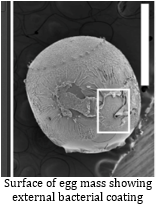 Since its accidental introduction into the Allentown, Pennsylvania area in the late 1990’s, the brown marmorated stink bug (BMSB), Halyomorpha halys, has established itself as a severe pest across much of the eastern United States. Much to farmers’ dismay, most conventional management techniques, including insecticides, do not effectively control the insect. This fact spurred the development of a working group in 2012 to research potential management strategies for the brown marmorated stink bug. The work of Chris Taylor investigating the importance of BMSB symbionts was part of this research. Taylor’s research focused on the relationship between the brown marmorated stink bug and a species of bacteria (Candidatus Pantoea carbekii) that resides in the insect’s gut, as well as on the surfaces of BMSB egg masses. He conducted a series of laboratory experiments looking at symbiont acquisition, nymphal survival, and fecundity under a variety of conditions. Are brown marmorated stinkbugs reliant on the bacteria smeared on egg mass surfaces? Previous research has shown varying levels of dependence on symbionts by different stink bug species, who obtain these symbionts be feeding on bacteria smeared on the surface of the egg mass. This resulted in the need to first confirm the level of symbiont reliance by the brown marmorated stink bug. To do this, aposymbiotic insects (those without symbionts) were generated by surface sterilizing egg masses using bleach and subsequently rearing these insects through all life stages. Survival, development time, and egg mass production was tracked and compared to stink bugs allowed to obtain their symbiont. Results indicated that BMSB is heavily reliant on its symbiont, showing significantly reduced survival, increased number of days to developmental peak, and reduced fecundity in aposymbiotic insects. Does environmental stress impact symbiont transmission or survival after transmission? Previous research has shown that some insects lose their symbionts under higher temperature conditions. In the case of BMSB, the effects of heat on the symbionts present both on the egg mass surface, and within the gut of the insect, had never previously been studied. To determine the effects of temperature on egg mass surface symbionts, egg masses were exposed to three fluctuating temperature conditions meant to mimic different mid-Atlantic summer conditions. The temperatures were classified as ‘normal’, ‘above average’ and ‘extreme’. The egg masses were exposed to one of these three temperatures, and returned to optimal rearing conditions as soon the eggs hatched. Hatch rate was calculated for each egg mass, and the ‘extreme’ condition was found to significantly reduce hatch rates and was therefore discarded. Hatch rate, survival, and percent inoculation were calculated for the ‘normal’ and ‘above average’ conditions, and no effect of temperature on the symbiont was detected. Next, Chris wanted to look at symbiont survival within the gut under heat stress. He settled for comparisons as 25 versus 30 degrees Celsius (mimicking previous studies) due to an inability to use the fluctuating conditions chambers for extended periods of time. Looking at the effects of these two temperatures on symbionts within the gut, a significant decrease in survival over time was seen in insects exposed to the higher temperature that were allowed to obtain their symbionts at birth. Additionally, decreases in fecundity were seen in the bugs at the higher temperature, as well as a drastic reduction in the number of bugs that still had their symbionts as adults. Results from these experiments indicated that the symbionts present on the surface of the egg mass are not likely affected by heat stress, but that heat stress over time has the potential to kill the symbiont within the gut of the bug. Can antimicrobial products remove the symbiont from the egg mass, and can this be used as a possible management strategy? The final experiments investigating the relationship between the brown marmorated stink bug and its’ gut symbionts addressed the potential for exploiting this relationship as a possible avenue of BMSB control. Using commercially available products with antimicrobial properties, Chris treated the symbionts on the egg mass surface like any other field crop disease, and tested to determine if any of these products had the potential to sterilize the egg mass surfaces. A total of six products were tested, and nymphs from each treatment were screened for the presence of gut symbionts. Of these six products, three were found to significantly reduce the number of BMSB able to obtain their symbiont from the egg mass surface. The findings of Chris Taylor’s research contribute much to the understanding of the complicated relationship between this insect and its symbionts. Future research is needed to determine sterilization efficacy of these products in a field setting, and to tease apart the different abiotic conditions that may limit BMSB’s host range. Further Reading: Taylor CM, Coffey PL, DeLay BD, Dively GP (2014) The Importance of Gut Symbionts in the Development of the Brown Marmorated Stink Bug, Halyomorpha halys (Stål). PLoS ONE 9(3): e90312. doi:10.1371/journal.pone.0090312  About the Author: Veronica Johnson is a Master’s student whose research focuses on determining the role of different post-harvest litter management practices in the degradation of Cry proteins in genetically modified Bt corn. She is also looking to determine the effects of winter temperature and precipitation on both tissue decomposition and protein degradation under these various litter management practices. In the mountains of California there are 9 species of millipede in the genus Motyxia, that do something no other millipede on earth can do. Every night they emerge from underground burrows and begin to glow with a constant soft blue-green light, and until recently no one knew why. While emitting light makes Motyxia unique among the millipedes it is hardly novel in the animal kingdom, and animals do it for a variety of purposes. Anglerfish in the deep sea glow to attract prey, but Motyxia are vegans. Fireflies flash with light to attract mates, but Motyxia, like all members of the order Polydesmida, are completely blind, so they aren’t searching the night for a glowing partner. While working at the University of Arizona Dr. Paul Marek set out to solve this mystery. Bioluminescent millipedes are exclusively found along the foothills of the Sierra Nevada, Tehachapi, and Santa Monica mountain ranges in Southern California. They live in majestic live oak and giant sequoia thickets, where they feast on decaying plant material and are sadly eaten in turn by rodents, centipedes, and phengodid beetles. These gentle and slow moving creatures appear to lack any defense, and glowing seems like the opposite of camouflage. It seems like they’re asking to be eaten! It turns out that glowing isn’t the only shocking chemical reaction taking place in these tiny legged bodies, they’re packed full of the deadly poison cyanide. Marek hypothesized that glow of these millipedes warns potential nocturnal predators that they will be an unpleasant meal, just as the bright colors of a wasp or a poison dart frog warns predators in the daytime to stay away! Scientists call the presence of warning signals like these aposematism. To test his theory of the function of bioluminescence, Marek created millipedes out of clay, painted some of them with glowing paint, and placed them in Motyxia habitats. After a night the clay millipedes were collected and checked for signs of predation. Rodents avoided attacking the glowing clay millipedes, indicating that rodents know to avoid the green glow and it’s accompanying mouthful of cyanide. Furthermore, at higher elevations the rodent populations are greater and more diverse, which means there is a much higher risk of being devoured by a predator. Over time the millipedes living at higher elevations have coped with this disadvantage by evolving brighter bioluminescence than those living at low elevations. This brighter and stronger warning keeps hungry rodents at bay! How they produce their light remains a mystery... Video courtesy of Marek Lab Further Reading:
Marek, P.E., D.R. Papaj, J. Yeager, S. Molina, W. Moore. (2011). Bioluminescent aposematism in millipedes, Current Biology, 21, R680-R681. Pieribone, V., & Gruber, D. F. (2005). Aglow in the dark: The revolutionary science of biofluorescence. Cambridge, MA: Belknap Press of Harvard University Press. About the Authors: Gussie Maccracken is a PhD. student in the Mitter Lab at the University of Maryland and the Labandeira Lab at the Smithsonian Institution National Museum of Natural History. She studies plant-insect interactions in the Late Cretaceous fossil record of North America. Peter Coffey is a Master's student whose research focuses on using cover crops to optimize sustainable farming economics. His current projects using lima beans and eggplant as model systems focus on plant nutrition, weed suppression, influences on pest and beneficial insects, and crop yield value. Follow him on twitter at @petercoffey. Follow Paul Marek on twitter at @apheloria.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290






 RSS Feed
RSS Feed




