 Ever wondered what creatures are lurking in your murky backcountry drainage ditches? Well, so has freshly-vindicated Dr. Alan Leslie. Through months of trudging through these mucky landscapes, he has sought to bring to light what is happening in the turbid depths of these ditches. Not only does the soil within contain a significant proportion of the Earth’s diversity, but these specialized environments are formed in juxtaposition of constantly fluctuating aquatic ecological systems and soil chemistry composition. The habitat acts as home to many critters that provide critical support for our agricultural food systems, energy cycling, and often serve as indicators for water quality. On the Eastern Shore of Maryland, 40% of the poorly drained land surface is used for agriculture, which places these ditches at an intersection of a significant proportion of water eventually trickling into our streams, rivers, and oceans…and drinking water. Think of these ditches not just as sporadic texture added to the otherwise flat agricultural landscape, but acting mitigation wetlands serving to reduce leaching of herbicides and pesticides; promote denitrification; and retain nutrients where they are most needed. The community mostly consists of organisms that are able to thrive in less than pristine conditions faced with constantly changing water volumes and chemical inputs. These impaired streams are brimming with different ecosystem engineering taxa that work to do similar jobs, even in the face of constant construction and deconstruction of the environment.
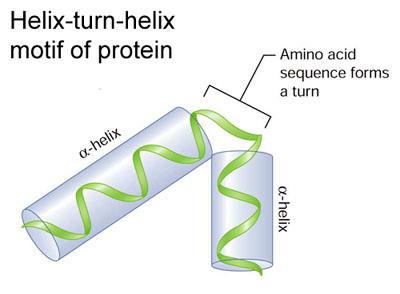
So who lives in the neighborhood? Alan discovered that 92.5% of everyone in the local ditch community lives at the water-sediment interface, right on beachfront property. Is it water quality that determines who settles down in a ditch?Surprisingly, no. Alan found that these burrowing invertebrate inhabitants did not correlate with water quality but did correlate with the size of the ditch. Effects of Pesticides on Honeybee Metabolic Physiology By: Steven Cook PhD  Dr. Steven Cook, Research Entomologist at USDA-ARS Bee lab, has shown that blends of pesticides can essentially ‘throw a wrench’ into a bee’s metabolic processes. He explained that constant detoxification might come at a high price. In order to keep the energetic budget balanced one or more basic biological functions (immunity, digestion, physical activity and basal metabolism) must compensate. Where exactly does the energy come from though? To calculate the cost of pesticides, Dr. Cook looked at the effects of commonly used pesticides and miticides on the metabolic physiology of caged nurse bees. Bees were exposed to different groups of compounds found in agricultural settings. One round of experiments focused on 2 controversial neonicotinoids, imidacloprid and clothianidin. Three variables were measured: 1) body weight, 2) metabolic rates (CO2/O2) via a technique calledrespirometry and 3) concentrations of two key nutritional components, lipids and proteins. As a worker bee’s role in the hive changes so, too, does its nutritional requirements. Thus measurements were taken at these normal transition phases (days 4, 7, 14 and 21). Dr. Cook found that physiological effects of the neonics were both compound and dose dependent. While many details can be gleaned from the graphs below, here are two interesting findings: by day 14 both compounds (at high doses) 1) drastically reduced protein content and 2) increased lipid content. GUY1 is developmentally regulated and is expressed in the embryonic stage of the mosquito life cycle. Once expressed, GUY1 is thought to bind to DNA, due to its specific helix-turn-helix shape, a structural domain commonly associated with DNA binding capabilities. The binding of GUY1 would then allow the expression of other genes, possibly involved in sex determination or dosage compensation. Expression of GUY1 in female mosquitoes results in total lethality by the early larvl stage; a phenotype which greatly skews the sex ratio of a population. By understanding the processes which causes this phenotype in mosquitoes, Dr. Criscione hopes to develop tactics to control the sex ratio in mosquito populations. This knowledge is critical to programs which use the release of sterile males (Sterile Insect Technique, SIT) or the release of insects carrying a dominant lethal gene (RIDL) to control mosquito populations. Sex-determination in the Anopheles mosquito is determined by the presence of specific sex chromosomes. Females possess two X chromosomes while males possess one X and one Y chromosome. By comparing sequence information between the male and female data sets, one can infer which regions are found exclusively on the Y, and therefore which sequences are associated with male specific genes. Using this conceptual approach, Dr. Frank Criscione was able to identify GUY1, a gene specific to the Y chromosome in mosquitoes, which is shown to generate a sex ratio bias when manipulated. Role of GUY1 in Mosquito Sex DeterminationThe Cytoneme Connection: Challenging our Knowledge of Paracrine SignalingAs we learned in colloquium earlier this semester, even the smallest arthropods have immense diversity in both body plan and development. According to Dr. Sougata Roy, a big contributing factor to this diversity is the ability of cells to communicate and induce changes in other cells. A major research interest of Dr. Roy’s is the function of cell structures called cytonemes in the development of Drosophila larvae. Cytonemes are well-known to be present during larval insect development and have been for quite some time, but until fairly recently, no one had researched their function. Belonging to a class of cellular protuberances called filipodia, cytonemes are filamentous structures known to be present in wing and eye imaginal disks in Drosophila and in the ovaries and embryos of several other invertebrates. Dr. Roy’s research suggests that these visually strange projections of the cell membrane play an integral role in paracrine signaling.
Paracrine signaling is generally known to involve releasing signal molecules directly into the extracellular fluid, which reach target cells slowly through diffusion. Dr. Roy believed that cytonemes may somehow facilitate this process, allowing the molecules to better home in on their target cells. Armed with countless Drosophila and red and green fluorescent biomarkers, he conducted several experiments to see if this could be the case. Beetles and Stress: Changing Local Climate One Tree at a Time
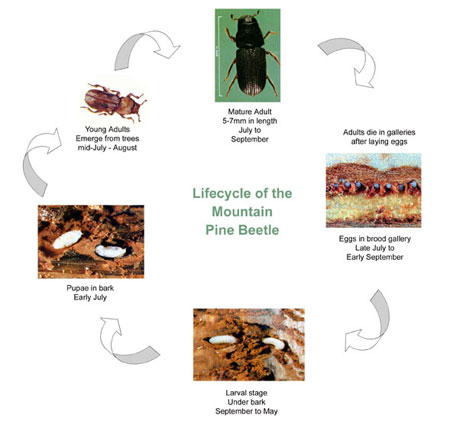
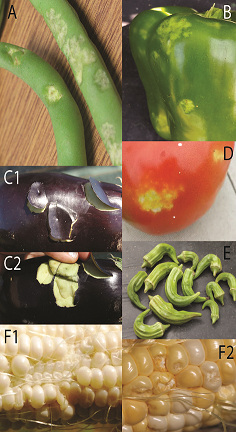
By: Melanie Vanderhoof PhD We often hear about the impacts of invasive species on local environments. However, an organism does not need to be invasive or large to have drastic effects at a landscape level. Mountain pine beetle (MPB) outbreaks in the south central Rocky Mountains exemplify the far-reaching effects that one small native species may have on entire ecosystems. Dr. Melanie Vanderhoof, currently an ORISE post-doctoral research fellow at the Environmental Protection Agency, investigated the impacts of these outbreaks on long-term energy and water fluxes in coniferous forests in Colorado and Wyoming. These variations to climate patterns can last for more than 40 years and coupled with global climate change, the dynamics of these outbreaks may also be altered. Post by: Thomas Pike and Ryan Gott Farm Field-Scale Tracking of BMSB: The Crop Circle of Life The brown marmorated stink bug (BMSB) (Figure 1) is an extremely destructive invasive pest that has wreaked havoc in crops and landscapes for over a decade. With over 170 different host plants, BMSB seems perfectly willing to exploit any of a number of different resources to survive. However, does it use all of these equally? Our speaker (and recent M.S. graduate) Emily Zobel asked this very question. She, along with faculty members Galen Dively and Cerruti Hooks, sought to determine which vegetables would be most attractive to BMSB at different times in the growing season. Emily planted several common vegetables (green beans, okra, eggplant, bell pepper, tomato and sweet corn) and looked for stink bugs and their feeding damage on the plants to determine which plants were most attractive at what time (Figure 2). She found that okra, bell peppers and sweet corn proved exceptionally popular for the stink bugs, with extraordinarily high numbers found on okra, while green beans, eggplant and tomatoes lagged significantly further behind in the preference race. However, when looking at fruit loss due to stink bug injury, the pattern is markedly different. Despite seeing the lowest numbers of stink bugs during the season, tomato damage was disproportionately high, with just over a third of the harvested tomatoes showing damage. Meanwhile, okra, despite harboring the overwhelming majority of stink bugs, lost a mere 20% of its fruit to stink bug damage. Of particular note in Emily’s research is the distinct lack of native stink bugs, such as the harlequin bug and the spined soldier bug, in the counts on plants. Despite noting that the rise of stink bugs in general is likely due to a reduction in the use of broad-spectrum insecticides, it seems that BMSB is the only beneficiary, with native stink bugs making up a measly 1% of the total stink bugs counted. Emily’s results have implications for scouting and managing BMSB. Better timing of scouting and application of control measures depends on the specific crop combinations being grown on a given farm. Particularly interesting is the strong attraction of BMSB to okra, showing it as a potential trap crop. About Thomas Pike and Ryan Gott:
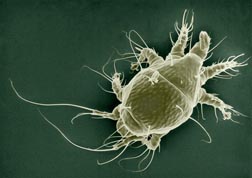
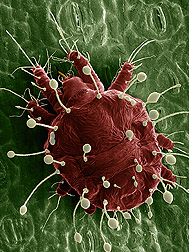
Thomas Pike is a third year Masters student in Paula Shrewsbury's lab conducting research on ornamental IPM. His primary research focus is on the effects of entomopathogenic fungi on the brown marmorated stink bug and their potential as a biological control. Ryan Gott is a PhD student in the lab of Bill Lamp. Ryan studies environmental toxicology and environmental risk assessment with a focus on developing biomarkers for chemicals that interact with ABC transporters. Post by: Brian Lovett and Andrew Garavito One Giant Leap for Mitekind When it comes down to it, scientists are not limited by their imaginations; they are limited by their tools of observation. Since the dawn of the scientific method, scientists have been in an arms race to invent finer, more advanced tools to probe the world around them. If we want to see distant galaxies more clearly, we send a telescope into space. If we want to discover elementary particles, we collaborate and build the world’s largest particle accelerator. What if we wanted to understand the morphology, behavior and phylogenetics of mites? Enter Dr. Ron “Oh My Goodness” Ochoa. He literally added another dimension to acarology (the study of mites). Before his ingeniously elegant application of Low Temperature Scanning Electron Microscope (LT-SEM) techniques to his field, mite researchers were busily removing microscopic mites from their natural setting and flattening them between two pieces of glass. By limiting their field to two dimensions, acarologists (those scientists who study mites) were left with ambiguous morphological traits for identification and almost no information on how the mites behaved naturally. Once Dr. Ochoa poured liquid nitrogen on the field of acarology, everything changed. Adapting techniques developed to count snowflakes (thank NASA for funding that study), Dr. Ochoa created beautiful, observable frozen landscapes of mites in their natural habitats, which are not only three dimensional, but also well-preserved. Stored in liquid nitrogen, Dr. Ochoa’s samples remain preserved to answer questions that scientists have today or may have thousands of years in the future. Armed with a Hitachi 4700LT-SEM, Dr. Ochoa tries to understand how the mites contribute to the ecosystem. As the only arthropod present in every ecosystem on our planet, they have serious potential to make an impact. Dr. Ochoa’s research is driven by the images he takes with his LT-SEM, which grant him the first glimpses of the vibrant microscopic community we step over every day. The word exciting doesn’t begin to explain the Leeuwenhoekian wonder Dr. Ochoa feels as the first human to see a mite mother protecting and feeding her children, to watch a mite feeding through the stomata of a plant, or, in a James Bond inspired epiphany, to understand the cryptic spiny projections of the female mites with a penchant for honey bee trachea. His research grants him ample opportunities to exclaim his notorious catch-phrase “oh my goodness.” With a curiosity that surpasses the names he gives the mites he discovers (Dr. Ochoa confesses that entomologists have stolen all of the good names for invertebrates), his research interests are seemingly boundless. During his entomology department seminar, Dr. Ochoa reminds the audience that it is hypothesized that every beetle, moth, ant, plant, animal and soil has a mite that is associated with it. At this very moment, these ambitious invertebrates are crawling all over you. They have accompanied us in our every accomplishment: riding along through collapses of civilizations and even into space. In his presentation, he dared molecular geneticists to match the resolution his techniques have on mite phylogeny and behavior, with newer 3D modeling techniques on his immediate horizon. Dr. Ochoa has, without a doubt, revolutionized acarology by taking and analyzing images that are as moving as they are pervasive: showing up on airline magazines and in advertisements worldwide. In the lab with Dr. Ochoa, one small pour of liquid nitrogen turned out to be one giant leap for our understanding of mitekind. Read more about his research and the methods used to take some of these amazing photographs here: Use of Low-Temperature Field Emission Scanning Electron Microscopy to Examine Mites About Brian and Andrew:
Brian Lovett is a PhD student studying mycology and genetics in agricultural and vector biology systems. He is currently working on projects analyzing mycorrhizal interactions in agricultural systems and the transcriptomics of malaria vector mosquitoes. Andrew Garavito is a first year Master’s student in Dennis vanEngelsdorp’s Lab. He is studying honey bees; with a focus on the diversity of pollen types brought in by foragers, and the health effects of different pollen diets. |
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290


 RSS Feed
RSS Feed




