Fall 2015 Colloquium: Dr. Steve Frank, Associate Professor at North Carolina State University11/19/2015
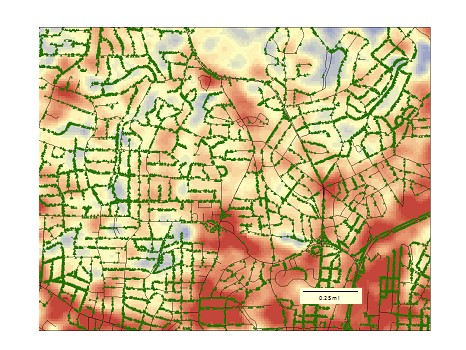
Increased Temperature Aids Pests in Overtaking Cities It has been observed for over one hundred years that plant insect pests such as scales, white flies, and thrips are more prevalent in urban areas than in forests. Many scientists have sought to understand this phenomenon with hypotheses on enemy release and abiotic influences. Enemy release relies upon the absence of natural enemies such as predators and parasitoids of pest insects in urban areas. In the resulting absence of enemies, populations of pests are allowed to build to extraordinarily high numbers. Abiotic influences, on the other hand, are aspects of the physical environment such as temperature that lead to increased population sizes. Dr. Steve Frank of North Carolina State University argues that the main driver in the high occurrence of pests in urban areas is not natural enemies but increasing temperatures in cities. Dr. Frank and his lab work with scale insects in Raleigh, North Carolina to elucidate the importance of temperature on pests, how pests impact tree health, and predicting effects of global warming. Does temperature affect the insect pests? Gloomy Scale, Melanaspis tenebricosa, is an excellent model for studying the importance of temperature on pest insects as it has a long history of localized high abundance in Raleigh. The earliest report on gloomy scale by Zeno Metcalf in 1912 heralded this insect as being the most important shade tree pest in North Carolina. Since that report, it has remained in high numbers in Raleigh but not in neighboring forest areas. Looking at heat maps of the area, Dr. Frank’s lab surveyed red maple trees within a gradient of temperatures in Raleigh and observed that gloomy scale abundance increased with temperature (Dale and Frank. 2014b). The question of why they were seeing this trend spurred additional investigation. (Left) Gloomy scales covering the tree’s branch making the bark appear rough and bumpy. Photo by Adam Dale. (Right) Zoomed in on the branch, you can see that each bump is an individual scale with its brown armor blending into the branch. The red dot in the middle is scale insect with its armor removed revealing the soft insect underneath. Photo by Matt Bertone The lab found that body size as well as egg production increased with temperature (Dale and Frank. 2014b). With increased egg production there is a corresponding increase in population growth, which helps the gloomy scale reach high densities. In short, because temperatures are higher in cities, gloomy scale is able to respond with increased body size, reproduction, and growth rate. Does temperature and pest abundance affect tree health? Dr. Frank’s lab wanted to investigate the effects of a possible interaction between warming and pests on tree health. To test this, they assessed the condition of all the red maple trees studied previously and then expanded the study to analyze 8462 trees inventoried by the city of Raleigh (Dale and Frank 2014a). Results showed that trees in hot locations, where scale abundance was therefore higher, were more than twice as likely to be in poor condition than trees in cold locations. The lab measured the water potential of the trees to help explain the physiological link between temperature and tree condition. Water potential in trees is linked to the ability to move water from the roots to the shoots. Dr. Frank’s lab found that water potential decreased with increasing site temperature, meaning that the trees were under higher drought stress in hotter areas (Dale and Frank 2014a). Linking temperature effects to both pest insects and tree health gives a holistic picture of the health and functioning of trees in urban landscapes. Can we predict the effects of global warming?  Historical red maple sample from an herbarium, which was assessed for scale abundance. (Youngsteadt et al. 2014. Global Change Biology) Historical red maple sample from an herbarium, which was assessed for scale abundance. (Youngsteadt et al. 2014. Global Change Biology) As urban areas are hot patches in the broad landscape, perhaps they can be used as proxies to look at the future of climate change. To tackle this idea, Dr. Frank’s lab used historical samples of red maple from herbarium collections, dating as far back as 1895. These samples were collected from rural areas around the southeast, and scales remain visible on them. The researchers found that the change in gloomy scale abundance with temperature variation was congruent across rural historical and modern urban samples (Youngsteadt et al. 2014). They also resampled trees at some of the historical sites and found that in most cases, the rise in temperature over the last several decades has led to an increase in gloomy scale abundance (Youngsteadt et al. 2014). Thus, it seems plausible that the relationships and patterns between scale insects and temperatures in cities could be used as an informative tool to predict potential outcomes of global climate change. What can we do today to help manage effects of temperature in cities? Urban areas around the world are becoming larger and hotter. As Dr. Frank’s lab has shown, the increase in temperature leads to more pest outbreaks, and these pest outbreaks have negative consequences for ecosystem services such as carbon sequestration and air purification. As temperature increases, cities will need to consider how to manage the effects on urban trees. For example, pest outbreaks may start occurring earlier in the season (Meineke et al. 2014), so scouting for pests should be increased. Cities should also try to plant more tree species and varieties that are resistant to the effects of increasing temperature on pest outbreaks and water stress. This may mean breeding or genetically modifying new varieties for cultivation. Concluding Thoughts In a broader sense, the decrease in urban forest canopy cover will have many negative effects both on wildlife and on humans. Studying urban trees will help identify problems, and potentially elucidate future effects of climate change. These effects threaten not only urban trees but also natural areas; therefore, understanding the impact of temperature on urban trees can help predict and prepare for the future of climate change across temperature gradients in landscapes. References: Dale A., S. Frank. The Effect of Urban Warming on Herbivore Abundance and Street Tree Condition. Plos One. 2014a. Dale A., S. Frank. Urban warming trumps natural enemy regulation of herbivorous pests. Ecological Applications. 2014b. Meineke E., R. Dunn, S. Frank. Early pest development and loos of biological control are associated with urban warming. Biology Letters. 2014. Youngsteadt E., A. Dale, A. Terando, R. Dunn, and S. Frank. Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Global Change Biology. 2014. About the Authors:
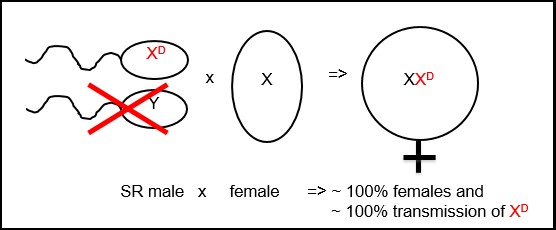
Jessica Grant is a master’s student currently working on kudzu bug (Megacopta cribraria) pest management. For more information on her work see this site mdkudzubug.org Aditi Dubey is a Ph.D. student looking at the effects of neonicotinoid seed treatment in a three-year crop rotation. Stalk-eyed flies reveal more than meets the eye - investigating sexual selection, speciation & genomic conflict Stalk-eyed flies are another one of nature’s curious and fascinating examples of biodiversity and an extreme result of sexual selective forces. These insects are true flies (Diptera) in the family Diospidae, typically distinguished by their unique eye stalks, from which they get their name. Dr. Gerald Wilkinson of the biology department at the University of Maryland has been conducting research on how genetic mechanisms may influence the outcome of evolution, specifically sexual selection, speciation and genomic conflict in stalk-eyed flies. All diospid flies have eye stalks, although some males display extremely long and seemingly disadvantageous stalks. When a species is sexually dimorphic, there is a physical difference between females and males, such as the difference in plumage between peacocks. In this case, the eye stalks are dimorphic with males having the longer eye stalks, and females having short eye stalks. While not all species of stalk-eyed flies are sexually dimorphic, sexual dimorphism has evolved independently several times in this genus. Based on Dr. Wilkinson’s research, the development of these intriguing eye stalks appears to be a product of sexual selection. The female flies favor mating with males with larger relative eye stalks to body size, pushing for directional selection for longer and longer eye stalks over evolutionary time. To test some predictions, Dr. Wilkinson artificially selected for both smaller eye stalk populations and larger eye stalk populations in the lab using Teleopsis dalmanni. Intriguingly, he found that after 20 generations, males from short eye stalk lines were producing only daughters and some males from longer eye stalk lines were producing predominantly sons. Dr. Wilkinson had stumbled onto X drive, an example of genomic conflict. Genomic conflict arises when certain genes selfishly try to push themselves into the next generation even at the expense of the host/population. In the populations of short stalk eyed flies, X drive influences spermatid elongation. Those that elongate properly contain the selfish driving element on the X chromosome (X) which in turn leads to a female biased population and spread of XD. Unchecked, this would lead to the eventual demise of the population. The discovery of X drive in T. dalmanni through artificial selection on male eye span, is a convincing catalyst for sexual selection. Because females that mate with XD carriers can lead to population extinction, there is pressure for females to avoid mating with these short stalk-eyed males. A preference for males with long eye-stalks can develop because 1) the population survives and 2) long eye-stalks are a good advertisement for the lack of XD. Because of this, Dr. Wilkinson predicted and found that eye span genes are X-linked and are in linkage disequilibrium with the driver element (i.e. short eye stalks genes stay close together with the driver element on XD). Sexual selection should eliminate drive except if recombination or imprecise female choice occasionally occurs.  Having unraveled some of the secrets to mating in stalk-eyed flies, Dr. Wilkinson turned his curiosity to another aspect. He was interested in identifying mechanisms for reproductive isolation. Which mechanism is more important, prezygotic isolation (e.g. differences in mating, sperm transfer, sperm survival, or fertilization) or postzygotic isolation (e.g. hybrid inviability or hybrid sterility) for the creation of new species? Using very similar species of southeast Asian stalk-eyed flies (cryptic species), Dr. Wilkinson and his lab determined that reproductive isolation increases with genetic distance and that those populations evolve postzygotic isolation rapidly, but prezygotic isolation relatively slowly over evolutionary time. In particular, hybrid males between closely related species were sterile. Dr. Wilkinson hypothesized this may be due to cryptic drive. This is where drive is hidden because another genetic element (a suppressor) is counteracting its effects. If the suppressor is removed the drive is suddenly active. Through breeding experiments he ultimately found the presence of both cryptic X and Y drive. Their suppressors were found on the non-sex chromosomes and were in the same genomic regions as factors that caused male hybrid sterility. So while stalk-eyed flies are a natural wonder to behold, it appears they have more to offer than meets the eye. From them we have learned that genomic conflict can influence sexually selected traits and that reproductive isolation can evolve rapidly due to post-mating sexual selection and X drive. Much of this work is ongoing and there is more to learn about genomic conflict and drive system using diospids as model organisms. References: Baker, R.H., Narechania, A., DeSalle, R., Johns, P.M., Reinhardt, J.A. and Wilkinson, G.S. 2015. Spermatogenesis drives rapid gene creation and masculinization of the X chromosome in stalk-eyed flies (Diopsidae). Genome Biology and Evolution (in review) Baker, R.H. and Wilkinson, G.S. 2010. Comparative genomic hybridization (CGH) reveals a neo-X chromosome and biased gene movement in stalk-eyed flies (genus Teleopsis). PLoS Genetics 6(9): e1001121 Christianson, S.J., Brand, C.L., and Wilkinson, G.S. 2011. Reduced polymorphism associated with X chromosome meiotic drive in the stalk-eyed fly, Teleopsis dalmanni. PLoS ONE 6:e27254. Christianson, S.J., Swallow, J.G., and Wilkinson, G.S. 2005. Rapid evolution of postzygotic reproductive isolation in stalk-eyed flies. Evolution 59(4):849-857 Reinhardt, J.A., Brand, C.L., Paczolt, K.A., Johns, P.M., Baker, R.H. and Wilkinson, G.S. 2014. Meiotic drive impacts expression and evolution of X-linked genes in stalk-eyed flies. PLoS Genetics 10(5):e1004362 Rose, E.G., Brand, C.L., and Wilkinson, G.S. 2014. Rapid evolution of asymmetric reproductive incompatibilities in stalk-eyed flies. Evolution 68:384-396 Wilkinson, G.S., Johns, P.M., Metheny, J.D., and Baker, R.H. 2013. Sex-biased gene expression during head development in a sexually dimorphic stalk-eyed fly. PLoS ONE 8:e59826 Wilkinson, G.S., Christianson, S.J., Brand, C.L., Shell, W. and Ru, G. 2014. Haldane's rule is linked to extraordinary sex ratios and sperm length in stalk-eyed fles. Genetics 198:1167-1181 Wilkinson, G. S., Presgraves, D.C. and Crymes, L. 1998. Male eye span in stalk-eyed flies indicates genetic quality by meiotic drive suppresion. Nature 391:276-279 gAbout the authors: Jackie Hoban is a first year Master's student in Paula Shrewsbury’s lab, researching biological control of the invasive, wood-boring pest, emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Jonathan Wang is a PhD student in Raymond St. Leger’s lab. He is studying Drosophila immunity and fungal pathogenesis. Mengyao Chen is a Master’s student in Dr. Leslie Pick’s Lab. Her research focuses on segmentation genes in Brown Marmorated Stink Bug (BMSB, Halyomorpha halys). Her current work is looking for orthologs of pair-rule genes in BMSB, and studying their expression and functions using RNA in situ hybridization and RNAi. Evolution Occurring Before Our Very Eyes: Parasitoids Diverging Based on Host’s Food Source Parasitic wasps, or parasitoids, lay their eggs inside other insects or animals, and their larvae feed on that insect or animal once they hatch. Many parasitoids lay their eggs in the larvae of other insects, for example caterpillars. Caterpillars often have adapted to feed on specific plants, called “host plants,” that may have chemical defensive properties (allelochemicals) that the caterpillars are able to cope with and sometimes incorporate in to their own chemical defenses. When parasitoids use these caterpillars as hosts, they too must adapt to the host plant’s toxins. These phenomena create a very interesting relationship between host plant, host insect, and parasitoid. The plant tries to defend against the host using toxins, the caterpillar incorporates and becomes immune to those toxins, and finally the parasitoids must adapt to the toxins present in the caterpillar host to guarantee its larvae can survive inside the host and eventually use it as food. Dr. Karen Kester received her PhD from the University of Maryland under the guidance of Dr. Pedro Barbosa researching the ability of parasitic wasps to adapt to the particular food plants and plant toxins of their insect hosts Parasitic wasps are a diverse group containing over 750,000 species, but the mechanisms of the group’s diversification are relatively unknown. Parasitic wasps are associated with a number of life history traits that can promote rapid adaptation to new hosts with specialized diets. These traits may have led to their incredible diversity. To test her hypotheses, Dr. Kester chose to focus on Cotesia congregata, a larval parasitoid of sphingid moths, including Manduca sexta caterpillars. These caterpillars have the ability to incorporate plant toxins into their own defense, for example the nicotine found in tobacco. Using field sites in multiple farms, and caterpillars feeding on either tomato or tobacco plants, she found that wasps localized to tomato-feeding caterpillars did poorly when made to parasitize tobacco-feeding caterpillars. On the other hand, wasps localized to tobacco fields had adapted to the nicotine toxins in the plants and the caterpillars and were able to cope with the toxicity. Dr. Kester determined that parasitoids can become genetically adapted to parasitize hosts found on locally abundant food plants (Barbosa et al, 1991; Kester & Barbosa, 1994). Dr. Kester went on to become a professor of Biology at the Virginia Commonwealth University where she has furthered her research on tritrophic interactions, first exploring Cotesia wasp adaptations to host – food plant complexes using both Manduca sexta and Ceratomia catalpae, important pests of tobacco and catalpa trees, respectively. Tobacco plants and catalpa trees differ in the types of chemicals they contain, and it was of interest to determine if these chemicals had any effect on the parasitoids of the two caterpillars. Dr. Kester determined that the wasps associated with tobacco-feeding caterpillars and catalpa-feeding caterpillars were genetically different, with a 2% sequence divergence in their mtDNA (Jensen et al, 2002; Kester et al, 2015). In fact, the genetic data revealed that the Cotesia wasps specializing on the two caterpillar types comprised two separate lineages with limited gene flow. This discovery raised additional questions regarding whether the two types of Cotesia could be considered different species, and led to another series of experiments. The primary question was if the differences were due to factors occurring before fertilization (prezygotic) or factors occurring after fertilization (postzygotic). Some prezygotic factors measured were the male response to female pheromones and male acoustic signals. Differential adaptations to host and food plants, as well as hybrid inviability are examples of some postzygotic factors that were explored. Hybrid inviability would occur if the two types Cotesia mated and had offspring that died before reaching adulthood. This would indicate that the two Cotesia wasps were so different that they could not successfully reproduce; this is called “reproductive isolation” and is an important part of the evolution of separate species. Results showed that fewer males responded to the pheromone from the “other” wasps compared to their own females. For example, wasps found to parasitize caterpillars feeding on tobacco were less likely to respond to pheromones of those parasitizing catalpa-feeding caterpillars (Bredlau, 2011; Bredlau & Kester, 2015). Dr. Kester also showed that the courtship songs between the two wasps also differed in duration and frequency, although no significant differences were detected in mating success (Bredlau et al, 2013). Although there were no differences in the success of different wasps mating, first-generation sibling crosses between progenies of two different wasps were discovered to be inviable. Dr. Kester’s current work investigates the underlying mechanisms causing this inviability. Recent discoveries have led her lab to believe the inviability in the first generation cross is related to the absence of polydnaviruses in the inviable offspring. Polydnaviruses are obligate symbiotic viruses that have coevolved with a subfamily of a group of Braconid parasitic wasps, including Dr. Kester’s target species Cotesia congregata (Albrecht et al, 1994; Savary et al, 1999). Ongoing research by Dr. Kester is exploring the evolutionary role of polydnaviruses as an underlying mechanism of speciation in wasps relative to changes in their host immune response. For instance, these viruses can lead to lateral gene transfer between wasp and host species, which may play an important role in the evolution of nicotine tolerance in the wasps associated with tobacco-feeding caterpillars. With the addition of viruses into this complex system, Dr. Kester now considers these species interactions to be “multi-trophic”. Dr. Kester’s final message was that, “once you know your system well, the questions never end.” She intends to further research differences in acoustic signals between different parasitic wasps, as well as look at other host – food plant complexes of Cotesia congregata. Dr. Kester’s work reminds us that evolution is occurring all around us and that new species are diverging right before our very eyes. References: Albrecht, U., Wyler, T., Pfister-Wilhelm, R., Gruber, A., Stettler, P., Heiniger, P., ... & Lanzrein, B. (1994). Polydnavirus of the parasitic wasp Chelonus inanitus (Braconidae): characterization, genome organization and time point of replication. The Journal of general virology, 75, 3353-3363. Barbosa, P., Gross, P., & Kemper, J. (1991). Influence of plant allelochemicals on the tobacco hornworm and its parasitoid, Cotesia congregata. Ecology, 1567-1575. Bredlau, J. (2011). Investigation of pre-and post-zygotic reproductive barriers between two host-plant complex races of the parasitic wasp Cotesia congregata (Say)[Hymenoptera: Braconidae]. Bredlau, J. P., Mohajer, Y. J., Cameron, T. M., Kester, K. M., & Fine, M. L. (2013). Characterization and generation of male courtship song in Cotesia congregata (Hymenoptera: Braconidae). PloS one, 8(4), e62051. Bredlau, J. P., & Kester, K. M. (2015). Pre-and Postzygotic Barriers to Reproduction Between Two Host-Foodplant Complex Sources of the Parasitic Wasp, Cotesia congregata (Hymenoptera: Braconidae). Annals of the Entomological Society of America, sav089. Jensen, K. M., Kester, K. M., Kankare, M., Brown, B. L. (2002) Characterization of microsatellite loci in the parasitoid, Cotesia congregata (Say) (Hymenoptera Braconidae). Molecular Ecology Resources. 2(3): 346-348. Kester, K. M., & Barbosa, P. (1994). Behavioral responses to host foodplants of two populations of the insect parasitoid Cotesia congregata (Say). Oecologia, 99(1-2), 151-157. Kester, K. M., Eldeib, G. M., & Brown, B. L. (2015). Genetic Differentiation of Two Host–Foodplant Complex Sources of Cotesia congregata (Hymenoptera: Braconidae). Annals of the Entomological Society of America, sav088. Savary, S., Drezen, J. M., Tan, F., Beckage, N. E., & Periquet, G. (1999). The excision of polydnavirus sequences from the genome of the wasp Cotesia congregata (Braconidae, Microgastrinae) is developmentally regulated but not strictly restricted to the ovaries in the adult. Insect molecular biology, 8(3), 319-327. About the Authors:
Grace Anderson is a Master’s student in the Sultz lab at UMD and works on the evolution of Harvestmen. She is currently exploring the morphological differences between closely related species that vary greatly in mating behavior. Veronica Johnson is a Master's student in the Hooks lab working with genetically modified corn. She is currently investigating the effects of different post-harvest practices on the persistence of toxic Bt proteins in senesced corn debris. |
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290







 RSS Feed
RSS Feed




