|
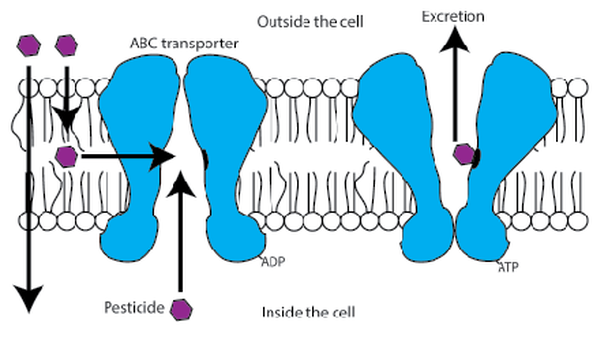
Post by Ryan Gott and Nathalie Steinhauer Colloquium this week was a special treat as Grace Kunkel defended her Master’s thesis “Investigatingin vivo honey bee toxicology and whole honey bee hive dynamics using fluorescent dyes.” Grace’s work focused on two different levels related to honey bee health: the individual honey bee and the entire honey bee colony. Collapsing colonies have been in the headlines since 2006, and with finger-pointing at many possible perpetrators. These potential causes of Colony Collapse Disorder (CCD) include bee parasites, bacterial and viral diseases, fungi, poor nutrition, and chemicals (but not cell phones!). While current research suggests that CCD is most likely caused by a combination of these factors, Grace decided to focus on how chemicals may be affecting the honey bees. While the most suspect chemicals include pesticides and fungicides, using these chemicals in experiments is complicated and expensive. As a solution Grace used dyes that were chemically similar to specific pesticides. This allowed her to not only save money, but also easily track the dyes though individual bees and colonies. When honey bees are exposed to pesticides, proteins called ATP-binding cassette transporters (ABCs) form the first line of defense by actively sequestering the chemicals in the honey bee’s gut to be excreted (Fig. 1). If these ABCs are inhibited, pesticides can build up to dangerously toxic levels in the honey bee’s blood. Grace found that both excretion by ABCs and inhibition of ABCs can be studied in individual honey bees using dyes that mimic the behavior of pesticides. If honey bees encounter ABC inhibitors in their environment, this could sensitize them to pesticides. The dynamics of a chemical in a whole colony, however, can be vastly different from those in an individual bee. When chemical exposure in a honey bee colony is explored, typically the chemical is applied directly into the hive through either sugar syrup or pollen patties. Grace questioned whether those two methods of chemical delivery were truly equivalent or if they lead to different distributions of the chemical in the colony. To explore this she once again used dyes as chemical surrogates. By incorporating dyes into syrup and patties, she could track their movements through each component of the colony, from honey to wax and from larvae to adult bees (Fig. 2). Dye delivered in sugar syrup gets stored and accumulates in the hive, while dye in pollen patties does not. Grace believes this shows that pollen patties are directly eaten by the bees and not stored, unless the patty is eaten while the bees are producing royal jelly. Royal jelly is a highly nutritious substance produced to feed future queens, so chemicals that end up in royal jelly could potentially affect future generations of bees. Grace’s findings have important implications for colony health. Behavior of expensive pesticides in a honey bee colony can be mimicked using similar dyes for a fraction of the cost. Delivery of chemicals through pollen patties equates to an “acute” exposure to a chemical, while the stored syrup would be a “chronic” exposure. This means the method of chemical delivery makes a big difference, and future researchers using actual pesticides in colonies should choose their delivery method wisely. About Nathalie: Nathalie Steinhauer is a PhD student working in the vanEngelsdorp lab. She studies the risk factors associated with beekeeping management linked to increased honey bee colonies mortality.
About Ryan:Ryan Gott is a PhD student in the lab of Bill Lamp. Ryan studies environmental toxicology and environmental risk assessment with a focus on developing biomarkers for chemicals that interact with ABC transporters. Post by Chris Taylor This week’s Colloquium was the first of the semester where a grad student in our department defended their dissertation. Bridget DeLay’s work, “Symbionts associated with the salivary glands of the potato leafhopper, Empoasca fabae, and their function when feeding on leguminous hosts” was a particularly interesting topic for me since I work with the symbionts of the brown marmorated stink bug. Mutualistic associations between animals and microbes have been documented in many taxa, so it is not surprising that these relationships are found in a diverse assemblage of insects. The field of insect-microbe interactions is a fast growing and exciting branch of entomology. With the relatively recent increase in the development and availability of genetic tools for identification and sequencing, more and more of these relationships are being uncovered. We have barely scratched the surface in terms of understanding the scope of these relationships and the roles that they play in the biology of the host organism. The microbial symbionts of insects usually have very intimate relationships with their hosts. Therefore, these symbionts are usually obligate (association is required for survival), vertically transmitted (parent to offspring), and have a reduced genome. They have a variety of functions, one of the most prominently studied being their role in diet supplementing. Most blood, xylem and phloem feeding insects have symbionts, which aid the host by providing the essential amino acids or vitamins that their nutrient-deficient diets cannot provide. Most described symbionts have been found in the guts of their hosts, but recently they have also been isolated from the salivary glands of some insects. The potato leafhopper is a highly polyphagous, native agricultural pest that causes a condition known as ‘hopper burn’ (here and here), which causes yellowing and stunting of the plants on which it feeds. Although Paul Buchner, one of the most well known researchers of insect-microbe interactions, referred to the Auchenorrhyncha (the suborder of insects to which the leafhoppers belong) as a “fairyland of insect symbiosis,” Bridget was the first to look at a cicadellid leafhopper to determine its symbionts. She identified two: a species of Wolbachia and a species of Sulcia. By using antibiotics to produce aposymbiotic (symbiont-free) leafhoppers, she investigated survivorship, fecundity, and nymphal survival when the symbionts were not present. She determined that both nymphal survival and survivorship were decreased when the symbionts were removed, indicating that the symbionts are necessary for success of the host leafhopper. Bridget also wanted to look at what role, if any, the symbionts played in feeding on plants by the leaf hopper. She allowed symbiotic and aposymbiotic leaf hoppers to feed on alfalfa and fava beans, and showed that symbiotic leaf hoppers reduced photosynthesis rates in alfalfa and fava beans as well as transpiration rates in alfalfa. In another study, she applied plant hopper saliva to the stems of plants and data suggested that the symbionts may actually suppress wound response pathways in the plants while the leaf hoppers feed. Bridget sequenced the 1st transcriptome data for cicadellid salivary glands that is now available in GenBank (see picture citation below). This work provides an insightful look into the intimacy of these insect-microbe interactions. Her research suggests the microbes aid the leafhopper in its feeding and contribute to the damage the plant endures: a very novel and understudied aspect of insect-microbe biology. With the advent of genetic identification tools, our knowledge of insect-symbiont interactions will continue to increase. This also opens up the possibility to exploit these symbiotic relationships for the management of pests like the potato leafhopper. More and more work, Bridget’s included, is showing that these associations are vital to the success of the host insect, and the disruption of this relationship may turn out to be a useful management tool for pest insects About Chris:
Chris Taylor is a PhD student with focal areas in IPM and insect-microbe symbioses. He studies the brown marmorated stink bug (BMSB), and the focus of his research is on understanding the relationship between BMSB and its gut symbionts to determine whether exploiting this relationship is feasible in management programs. Post by Brian Lovett In a world that wants to bring an end to the malaria-causing parasite Plasmodium, Dr. David O’Brochta’s lab is busily working to create more of it. By carefully altering the genome ofAnopheles stephensi, a mosquito that carries Plasmodium, he is turning these sinister insects intoPlasmodium factories. Though most people find the idea of a mosquito’s salivary gland packed full with Plasmodium sporozoites terrifying, it forms the foundation of a promising malaria vaccine to the researchers at Sanaria Inc. In his research overview seminar at the UMD Entomology department colloquium, Dr. O’Brochta details his history of using unusual means to achieve useful ends. At the heart of his research is an aim that is as old as the field of genetics: develop a “seamless understanding” of the relationship phenotype has with genotype. With the dawn of genomics and transcriptomics, the scientific world has accumulated much more information on genotype than on phenotype. Without a proper connection between these strings of genetic letters and the characteristics we see from them, we are living in a world where we know more than we understand. Drawing the connection back from genotype to the phenotype is called reverse genetics, and these connections are driven by genetic technologies. Dr. O’Brochta has developed transposon-based genetic technologies to try to connect the dots between the vast genetic information researchers now have on mosquitoes and how these genes work together to create a mosquito. Dr. O’Brochta was careful to point out that he did not re-invent the wheel: Barbara McClintockwas the first to characterize the action of transposons in her Nobel prize winning experiments on corn, and generations of Drosophila researchers have been pioneering and testing transposon-based genetic engineering for decades. Rather, Dr. O’Brochta is “making it work in new systems.” His new system is the mosquito, and his innovations using these transposons have some amazing applications. Transposons are small genetic sequences that have the ability to jump around genomes carrying with them whatever sequences they contain. Dr. O’Brochta describes transposons simply as chassis with an engine. The engine is the enzyme that allows the transposon to move about, and the chassis is the sequence that is being moved. Like cargo, Dr. O’Brochta has loaded reporter sequences onto the piggyBac transposon. The reporter sequences respond to their location along the genome and report back, often with an observable fluorescence. This system isn’t particularly useful when the transposon is constantly using its engine to jump around in the genome, so after the reporter transposon finds its home in the mosquito’s genome, the mosquitoes are crossed with wild-type mosquitoes to remove the transposon’s engine. The mosquitoes that result from this cross end up with a reporter sending out information about their precise location in the genome. With manipulation of the strength of the promoter and the splice site affinity, Dr. O’Brochta can use these reporters to discover enhancer sequences hidden along the genome and find spaces between the exons that make up genes. These studies reveal, with incredible detail, where, when and what is transcribed along the genome. The pictures below show a mosquito enhancer that was found to only drive expression of the reporter in the abdomen of female mosquitos. The identification of this enhancer allows scientists to study and express genes in the same location and at the exact same moment in mosquito development. This allows unprecedented control over transgene expression in mosquitoes. 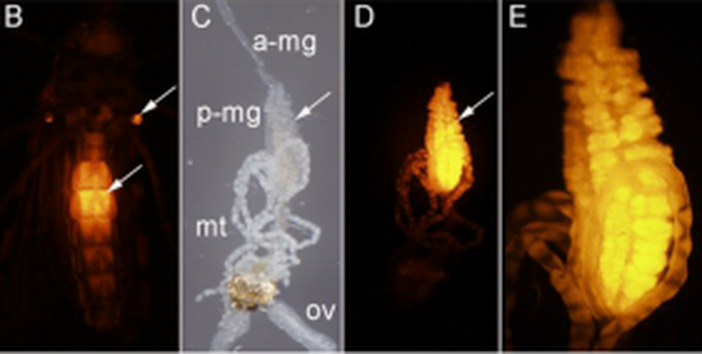
B – ventral view of an adult female under UV light showing enhancer-driven reporter gene expression in the abdomen (arrow) and halteres (arrow) (red fluorescence). C – dissected alimentary canal and ovaries (ov) of adult female shown in B; anterior midgut (a-mg), posterior midgut (p-mg), Malpighian tubules (mt) D - same as C but viewed under UV light to reveal reporter gene expression in the posterior midgut. E- higher magnification of the posterior midgut shown in D.
Dr. O’Brochta wants to use these techniques to “pop the hood and see what’s inside” of mosquito physiology and genetics. Already he has begun investigating the genes of mosquito hemocytes and salivary glands. Much can be learned about hemocyte gene expression, and many salivary gland genes have an unknown function. Due to the flexibility and high activity of the piggyBac transposon system he has developed, Dr. O’Brochta’s potential projects are only limited by the imagination. If you would like to learn more about his work, please visit his lab website. Brian Lovett is a PhD student studying mycology and genetics in agricultural and vector biology systems. He is currently working on projects analyzing mycorrhizal interactions of winter wheat and the transcriptomics of malaria vector mosquitoes
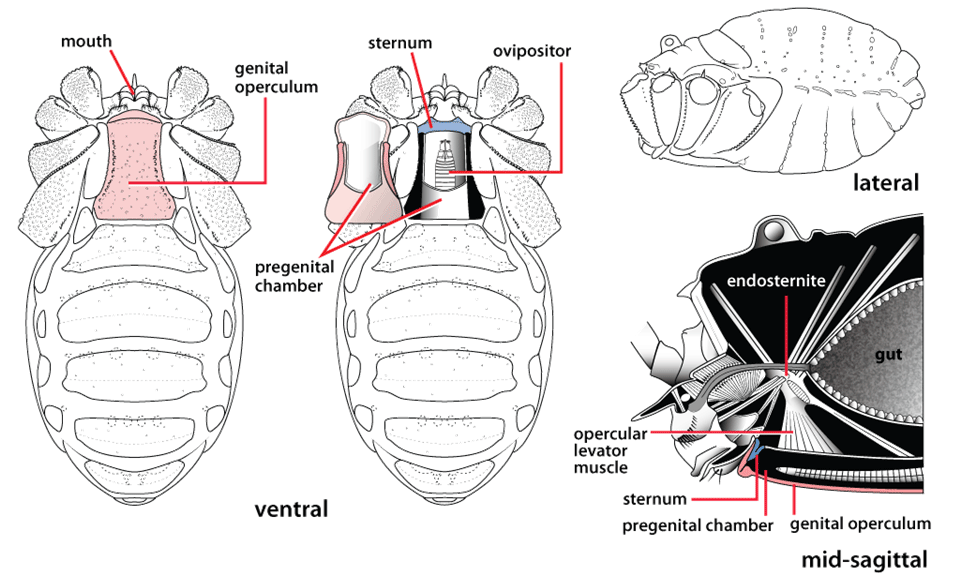
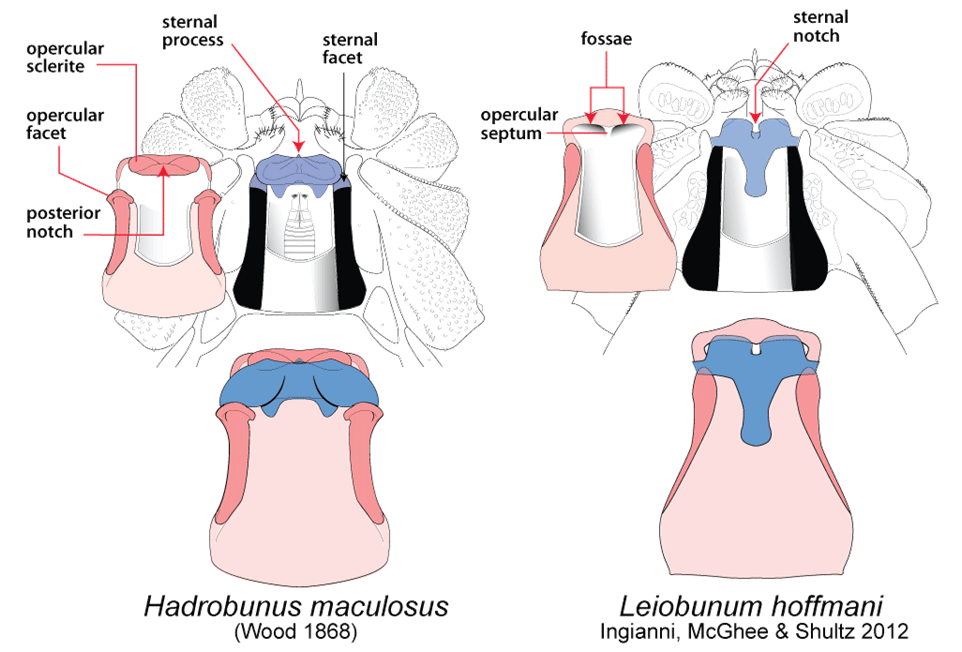
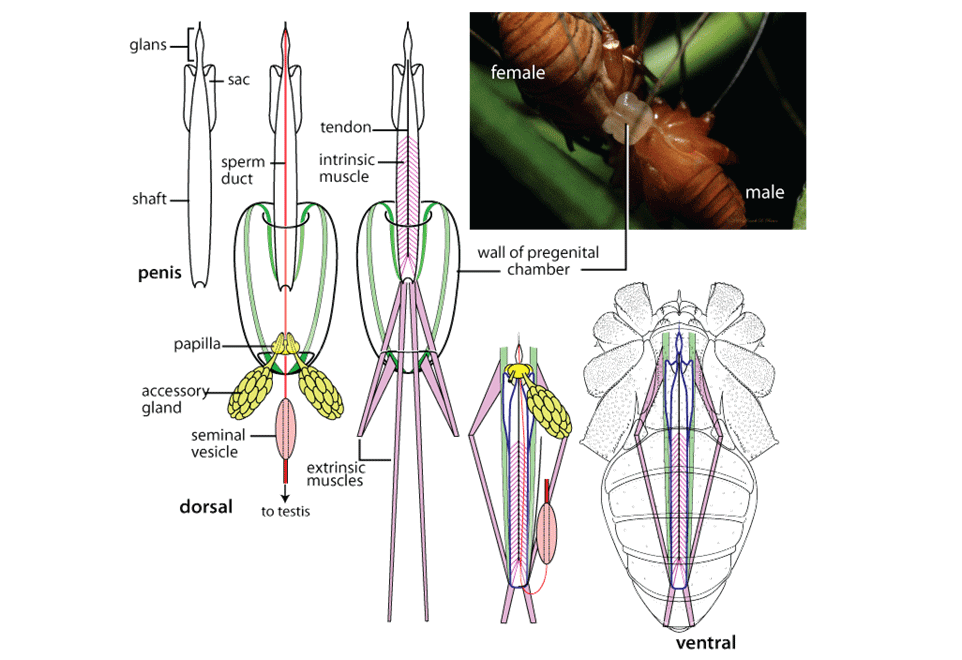
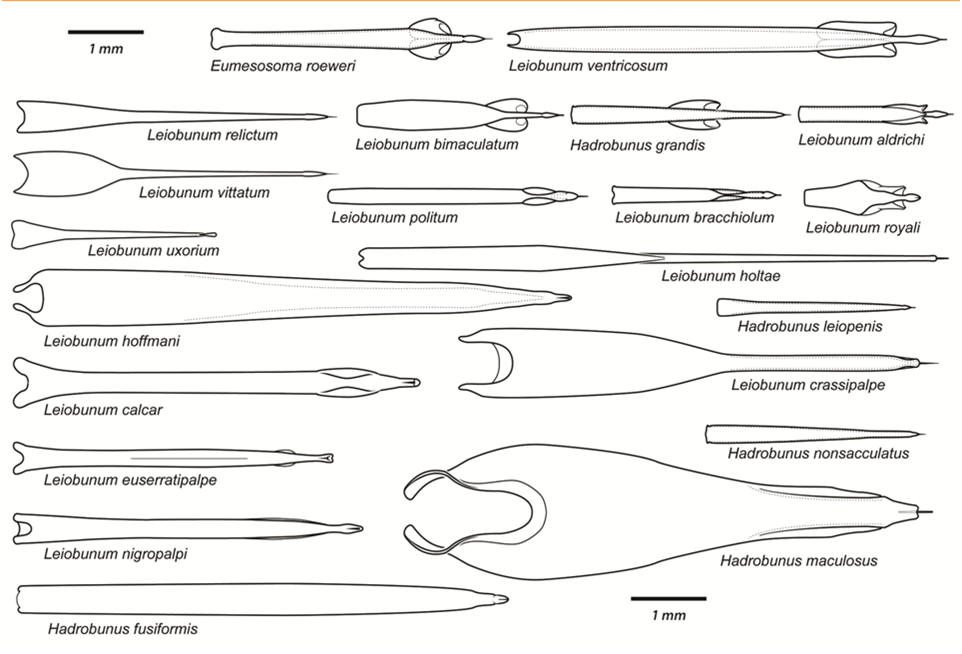
Post by Armando Rosario-Lebron Dr. Jeffrey Shultz, our resident arachnologist, gave us insight into the sex life and morphological diversity of harvestmen or Opiliones, also known as “daddy longlegs”. These arachnids are often ignored and thus provide an open territory for new research. A trek through the woods surrounding the Washington DC metropolitan area will often yield a few harvestmen. According to Dr. Shultz, some of these may be new species that have been overlooked even in the most populated areas of the Eastern United States. Your own back yard could be host to a “new” species of harvestman, undescribed by the scientific community. The Shultz lab now focuses most of their research on the genera Leiobunum and Hadrobunus. Until recently, Hadrobunus was thought to contain only two species, both first described in the 19th century until the recent additions of 11 new species by the Shultz lab. According to Dr. Shultz most species within the genus occur in the southeastern US, with a few lineages reinvading the north following the retreat of the glaciers The pattern suggests that the South served as a glacial refugium. However, what really makes the story interesting is the resulting diversity in the sex lives of both Leiobunum andHadrobunus, which suggests that there has been a hidden “sexual arms race” were males either coerce or entice females to mate with them. In many organisms females tend to “call the shots” in choosing a male that may meet standards for health or increase the chances of offspring and/or egg laying success with nuptial gifts. The healthy plumage of a peacock’s feathers for example may indicate health, “good genes” and a higher success rate for future offspring. This system attempts to ensure that the “best genes” are passed from generation to generation. However, as in all natural systems the rules can be broken and some males have found ways to bypass the filters to mating that females have put up. This drama of romance and coercion is the current focus of the research within the Shultz lab and is apparent within the morphology of harvestmen. Dr. Shultz gives us beautiful illustrations on the genitalia of harvestmen, all critical to the sexual arms race. The diversity within the group is vast – some males have large penises that may even be longer than the body. Other males may have special glands to entice the female to eat and mate. These become tools to penetrate the fortress of the female reproductive system in harvestmen. The females themselves may have bulwarks in the form of an armored operculum or flap covering their pre-genital opening near the mouth. These plates may be notched and sealed shut with immense force by muscles all attaching to an internal structure called an endosternite. As a testament to the strength of the plates, Dr. Shultz noted that during dissection removal of the plate may make a sudden audible pop when pulled open. In some females these may be further modified with the entire structure forming a unit sealed shut and wedged between two sternal plates, effectively locking the structure. Beyond that lies a worm-like ovipositor with a small opening that is the target that the male’s penis has to reach. She uses this ovipositor to both probe the soil and lay her eggs in the right location. The males in response have a penis that extends via hydraulic function. Similar to the diversity of the female defensive mechanisms the male penises vary in the number of muscles they have and the presence of special sacs they use to entice the female to mate. These sacs release a nuptial gift directly into the female's mouth. As she is eating the female will eventually open her operculum and the penis will enter the pregenital chamber. In some species this process of enticing or coercing the female may take hours, in others minutes. Dr. Shultz's research revolves around determining the evolutionary relationships between these harvestmen based on the presence or absence of these sacs. Eastern North America, Central America, Europe and Asia appear to be centers of biodiversity for harvestmen which led to the question: Are the harvestmen of eastern North America a monophyletic group or a phylogenetic mosaic with relationships across the globe? According to Dr. Shultz there is sufficient confidence in recent molecular data to show that most of the North American group is monophyletic or derived from a common ancestor and is not a phylogenetic mosaic. Graduate student Mercedes Burns in the Shultz lab explored a facet of this hypothesis via a phylogenetic analysis of the relationships between harvestmen, focused on the states of the presence or absence of nuptial sacs. In essence two methods were used. One assumed that the “ancestral” state of species is the presence of nuptial sacs, but these could be lost evolutionarily. The second method attempts to determine the rate of change from having sacs to not having sacs and overlays these over the relationships between species of harvestmen on a tree. The results provided evidence that, over time, female barriers were erected in response to males “cheating the system” and no longer using sacs with nuptial gifts. However there was insufficient evidence to suggest that lack of a sac also resulted in males morphologically forcing their way into the female operculum. Dr. Shultz is now collecting data on the strength of male penile protractor muscles and comparing that to female operculum muscles. His laboratory is also working on determining if there is a difference in the flexibility of the harvestmen penis and if this is correlated with an ability to force their way into the operculum. In the end, Dr. Shultz would aims for a “big picture” where he can test whether environmental conditions drive this sexual arms race and create shifts between enticement vs. antagonistic strategies in harvestmen. This may provide insights into sexual selection in other organisms and how changes in the environment can shift reproductive strategy. About Armando
Armando Rosario-Lebron is a Graduate Student within the laboratory of Dr. Cerruti RR Hooks. He is currently studying methods to augment natural enemies of Stink Bugs in agricultural systems. |
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290




 RSS Feed
RSS Feed




