|
Post by Brian Lovett In a world that wants to bring an end to the malaria-causing parasite Plasmodium, Dr. David O’Brochta’s lab is busily working to create more of it. By carefully altering the genome ofAnopheles stephensi, a mosquito that carries Plasmodium, he is turning these sinister insects intoPlasmodium factories. Though most people find the idea of a mosquito’s salivary gland packed full with Plasmodium sporozoites terrifying, it forms the foundation of a promising malaria vaccine to the researchers at Sanaria Inc. In his research overview seminar at the UMD Entomology department colloquium, Dr. O’Brochta details his history of using unusual means to achieve useful ends. At the heart of his research is an aim that is as old as the field of genetics: develop a “seamless understanding” of the relationship phenotype has with genotype. With the dawn of genomics and transcriptomics, the scientific world has accumulated much more information on genotype than on phenotype. Without a proper connection between these strings of genetic letters and the characteristics we see from them, we are living in a world where we know more than we understand. Drawing the connection back from genotype to the phenotype is called reverse genetics, and these connections are driven by genetic technologies. Dr. O’Brochta has developed transposon-based genetic technologies to try to connect the dots between the vast genetic information researchers now have on mosquitoes and how these genes work together to create a mosquito. Dr. O’Brochta was careful to point out that he did not re-invent the wheel: Barbara McClintockwas the first to characterize the action of transposons in her Nobel prize winning experiments on corn, and generations of Drosophila researchers have been pioneering and testing transposon-based genetic engineering for decades. Rather, Dr. O’Brochta is “making it work in new systems.” His new system is the mosquito, and his innovations using these transposons have some amazing applications. Transposons are small genetic sequences that have the ability to jump around genomes carrying with them whatever sequences they contain. Dr. O’Brochta describes transposons simply as chassis with an engine. The engine is the enzyme that allows the transposon to move about, and the chassis is the sequence that is being moved. Like cargo, Dr. O’Brochta has loaded reporter sequences onto the piggyBac transposon. The reporter sequences respond to their location along the genome and report back, often with an observable fluorescence. This system isn’t particularly useful when the transposon is constantly using its engine to jump around in the genome, so after the reporter transposon finds its home in the mosquito’s genome, the mosquitoes are crossed with wild-type mosquitoes to remove the transposon’s engine. The mosquitoes that result from this cross end up with a reporter sending out information about their precise location in the genome. With manipulation of the strength of the promoter and the splice site affinity, Dr. O’Brochta can use these reporters to discover enhancer sequences hidden along the genome and find spaces between the exons that make up genes. These studies reveal, with incredible detail, where, when and what is transcribed along the genome. The pictures below show a mosquito enhancer that was found to only drive expression of the reporter in the abdomen of female mosquitos. The identification of this enhancer allows scientists to study and express genes in the same location and at the exact same moment in mosquito development. This allows unprecedented control over transgene expression in mosquitoes. 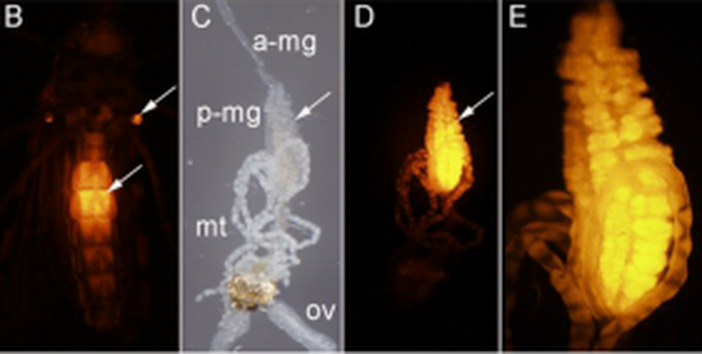
B – ventral view of an adult female under UV light showing enhancer-driven reporter gene expression in the abdomen (arrow) and halteres (arrow) (red fluorescence). C – dissected alimentary canal and ovaries (ov) of adult female shown in B; anterior midgut (a-mg), posterior midgut (p-mg), Malpighian tubules (mt) D - same as C but viewed under UV light to reveal reporter gene expression in the posterior midgut. E- higher magnification of the posterior midgut shown in D.
Dr. O’Brochta wants to use these techniques to “pop the hood and see what’s inside” of mosquito physiology and genetics. Already he has begun investigating the genes of mosquito hemocytes and salivary glands. Much can be learned about hemocyte gene expression, and many salivary gland genes have an unknown function. Due to the flexibility and high activity of the piggyBac transposon system he has developed, Dr. O’Brochta’s potential projects are only limited by the imagination. If you would like to learn more about his work, please visit his lab website. Brian Lovett is a PhD student studying mycology and genetics in agricultural and vector biology systems. He is currently working on projects analyzing mycorrhizal interactions of winter wheat and the transcriptomics of malaria vector mosquitoes
Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290

 RSS Feed
RSS Feed




