Fall 2015 Colloquium: Dr. George Dimopoulos of John Hopkins University School of Public Health12/21/2015
A Better Mosquito Malaria is a poorly named disease: literally meaning, “bad air,” it was born out of the miasma theory that disease was spread not by germs, but by a pollution of the air. Today, we know that it is mosquitoes, flying through the air, which spread this disease in a delicate dance between parasite (Plasmodium), vector (Anopheles mosquitoes) and host (humans). Malaria has been killing us since the dawn of civilization, so scientists are hard at work to throw this dance off-kilter. The most obvious solution is the one you implement with your hand whenever a mosquito lands on you: kill the mosquito. As a result, much of the work in vector biology aims to cut in on the dance between mosquitoes and humans; however, during the UMD Entomology colloquium this week, Dr. George Dimopoulos of Johns Hopkins University explained how he is trying to tip the scales where the mosquito and the parasite interact. 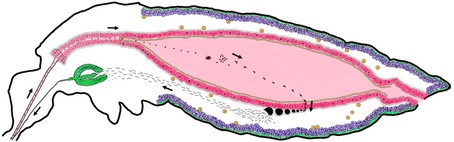 Figure 1: The developmental cycle of the malaria parasite in a mosquito. In this cross section of a mosquito, we can see how the parasites enter the mosquito through the proboscis (mouth) and imbed into the gut (pictured center, pink). After developing, the parasites burst into the mosquito blood and enter into the (green) salivary glands. From there, they are injected into a human host during biting. Image: Dimopoulos group. Figure 1: The developmental cycle of the malaria parasite in a mosquito. In this cross section of a mosquito, we can see how the parasites enter the mosquito through the proboscis (mouth) and imbed into the gut (pictured center, pink). After developing, the parasites burst into the mosquito blood and enter into the (green) salivary glands. From there, they are injected into a human host during biting. Image: Dimopoulos group. When Dimopoulos began investigating mosquito immunity, many found such work interesting, but impractical. Few could anticipate the exponential growth of the fields of molecular biology and genetics: in just 20 years, the number of known genes in a mosquito went from 13 to about 14,000. Scientists can now finely rewire the genes of insects, allowing Dimopoulos and his colleagues to “build a better mosquito:” one which does not carry the malaria parasite. In order to develop a better mosquito, it is important to keep in mind that the mosquito immune system is working with us to prevent malaria. While wild mosquitoes may ingest and spread many malaria parasites, the number of surviving parasites diminishes into the single digits in the gut of the mosquito (Figure 1). This bottleneck effect leaves the parasite vulnerable because at this point, the death of a few parasites in each mosquito would be the end of malaria. A mosquito may consume numerous parasites, but only end up with a couple surviving in its gut because its immune system fights hard to kill every last one. With the mosquito immune system as his ally, Dimopoulos engineered mosquitoes armed with enhanced immune systems able to annihilate invading parasites. He did this by combining genes already present in the mosquito. He increased the expression of a regulatory gene (Rel-2) from the IMD pathway (named the “Immune Deficiency” pathway because mosquitoes without this crucial pathway are susceptible to many pathogens), which turns on expression of multiple immune genes at the end of this pathway. By using a regulatory gene to attack the parasites on all fronts, Dimopoulos describes this method as similar to a “multi-drug therapy, by targeting the pathogen by multiple drugs; perhaps lessening the pathogen’s ability to evolve resistance.” He chose to combine this gene with the promoters (which determine when a gene is expressed) from two other genes: a protease (carboxypeptidase) or an egg production gene (vitellogenin). These genes are very highly expressed when a mosquito consumes blood, which is when the parasites enter the gut. This is also just before the bottleneck. These combinations of genes supercharge the immune system when the mosquito needs it most [1]. When Dimopoulos feeds these mosquitoes Plasmodium in the lab, the majority can successfully prevent the malaria infection. However, for this technique to work in the field, the Dimopoulos group wants to prevent infections entirely in all of the mosquitoes. To do this, they are creating hybrid strains expressing both the egg production and protease gene. They are also planning to try combinations with other immune genes to further enhance the immune system and bring infection down to zero. 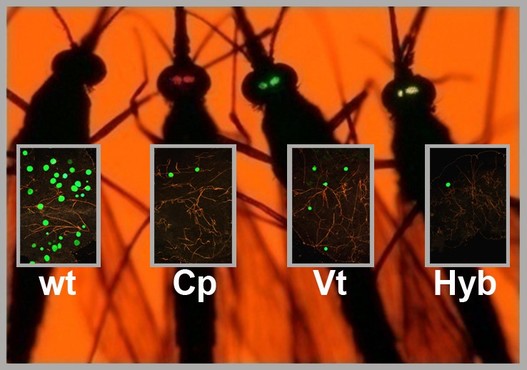 Figure 2: Four mosquitoes demonstrating their susceptibility to Plasmodium. Wt is an unmodified mosquito, Cp expresses Rel-2 driven by the carboxypeptidase promoter (red eyes), Vt has Rel-2 driven by the vitellogenin promoter (green eyes), and Hyb is a hybrid of both Cp and Vt. The green spots in the boxes over each mosquito represent their level of Plasmodium infection. Image: Dimopoulos group. Figure 2: Four mosquitoes demonstrating their susceptibility to Plasmodium. Wt is an unmodified mosquito, Cp expresses Rel-2 driven by the carboxypeptidase promoter (red eyes), Vt has Rel-2 driven by the vitellogenin promoter (green eyes), and Hyb is a hybrid of both Cp and Vt. The green spots in the boxes over each mosquito represent their level of Plasmodium infection. Image: Dimopoulos group. In lab experiments, Dimopoulos has found that his mosquitoes can gain resistance to malaria without a fitness cost, challenging the “dogma that says that it is impossible to make something fitter than an organism in nature.” He theorized that this is because the burst of immune system activity is limited to the time when blood is ingested; this happens to be a period of energy abundance for mosquitoes as they rest and digest their blood meal. Dimopoulos stressed the need to validate all his lab findings in field conditions at the Johns Hopkins MalariaSphere in Zambia. Recently, “the overall field of transgenic mosquitoes for control of vector borne diseases has received new enthusiasm” due to significant developments in biotechnology (such as novel gene drive systems). We have entered a translational phase where ideas and knowledge gained from basic research get applied, and when it comes to vector control involving mosquito immunity, Dimopoulos is leading the way. References:
1. Dong, Y., Das, S., Cirimotich, C., Souza-Neto, J. A., McLean, K. J., & Dimopoulos, G. (2011). Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog, 7(12), e1002458-e1002458. More by Dr. George Dimopoulous: Cirimotich, C. M., Dong, Y., Clayton, A. M., Sandiford, S. L., Souza-Neto, J. A., Mulenga, M., & Dimopoulos, G. (2011). Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science,332(6031), 855-858. Ramirez, J. L., Short, S. M., Bahia, A. C., Saraiva, R. G., Dong, Y., Kang, S., ... & Dimopoulos, G. (2014). Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. About the author: Brian Lovett is a PhD student studying mycology and genetics in agricultural and vector biology systems. He is currently working on projects analyzing mycorrhizal interactions in agricultural systems, the transcriptomics of malaria vector mosquitoes, and the genomes of entomopathogenic fungi. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290

 RSS Feed
RSS Feed




