|
Dr. Dawn Gundersen-Rindal is a researcher at the Invasive Insect Biocontrol and Behavior laboratory at the USDA ARS facility in Beltsville, Maryland. The goal of her team is to use biological, molecular, chemical and non-chemical techniques to develop novel, sustainable and cost-effective methods for the control of arthropod pests. They focus on invasive agricultural pests like the Brown Marmorated Stink Bug (Halyomorpha halys), medically important disease vectors like the Asian Tiger Mosquito (Aedes albopictus), and urban pests such as bed bugs (Climex lextularius). These pests in particular, have historically been difficult to control with some classes of traditional pesticides. Many of the pesticides available today are slow acting and persist in the environment for long periods of time. They are also indiscriminate, and will suppress beneficial arthropods, such as pollinators and natural enemies, and sometimes harm other organisms such as mammals, birds, and aquatic fauna. The advent of genetically modified crops allowed us to deliver toxins directly to the insects that were consuming the crop we wanted to protect. This directed approach protected the environment from broad applications of pesticides that were far more harmful to local ecosystems. However, they led to issues of resistance to the toxins used in GM plants and the potential of debris and the by-products from GM plants persisting the environment.
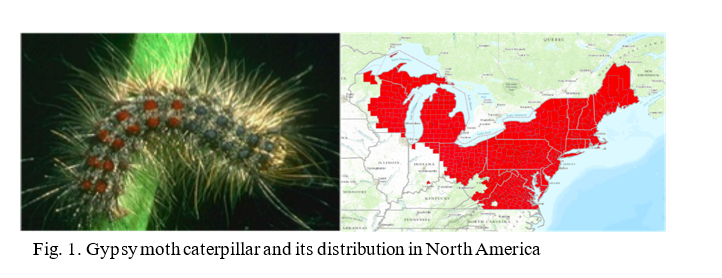
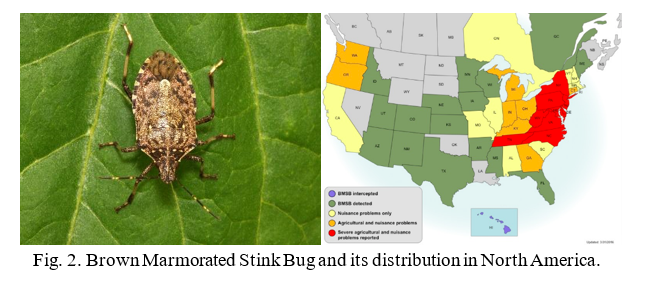
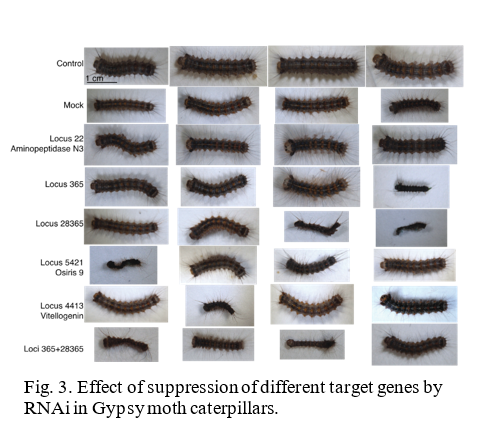
The lab is currently conducting RNAi research on two insects, the Gypsy moth (Fig.1) and the Brown Marmorated Stink Bug (BMSB) (Fig. 2). The European Gypsy moth (Lymantria dispar dispar) has been present in the US as an invasive pest since 1869, but has become a bigger threat with several recent accidental introductions and eradications of the Asian Gypsy Moth, (Lymantria dispar asiatica and other subspecies). The Asian gypsy moth is more destructive than its European counterpart due to the adult females having the ability to fly, allowing them to disperse much further, and the extensive number of trees and plants it defoliates. These traits are passed on to hybrid offspring of the two subspecies, further exacerbating this potential problem. BMSB, as mentioned previously, is a recent invasive that is an extremely damaging agricultural pest in the northeastern US. It has over 300 hosts and has caused billions of dollars in losses. It is also a nuisance pest as it overwinters within buildings. The USDA team focused on two requirements for developing RNAi control methods for these pests: 1) identifying suitable target genes and 2) finding an effective method for delivering dsRNA. The dsRNA is produced either through bacterial transformation (making bacteria produce mass quantities) or in vitro using PCR (using a machine to produce large quantities of the substance). The dsRNA is orally delivered through artificial diet in the lab. The minimum requirement for identifying target genes is a comparative transcriptome (a catalog of the messenger RNA expressed in a cell or group of cells), although a complete genome is preferred. The transcriptome used for the Gypsy Moth focuses on gene expression in response to midgut microbes. Preliminary trials did suppress some of the targeted gut genes in the Gypsy Moth, but this did not have a significant effect on development or mortality. Because RNAi can only suppress gene to a certain extent (maximum so far is 80%), it is necessary to identify genes important enough that partial silencing will cause severe damage. In the Gypsy moth, the most promising results came from two genes of unknown function, which led to a significant reduction in egg mass production. BMSB research is currently being conducted using two transcriptomes, and the whole genome is also being assembled. Although less dsRNA is required to kill BMSB with gene suppression than the Gypsy moth, delivering dsRNA is more difficult because it eats using a needle-like stylet, making an artificial diet or spray impractical. The team has developed a method to feed sufficient dsRNA to the BMSB for gene suppression, and is currently testing RNAi against different target genes in this species. These trials are currently in their initial phase and are expected to progress faster than the gypsy moth research due to the ease in causing mortality.
RNAi is an extremely promising pest management technique, but it is still in the preliminary stages of development. After a formulation is developed for field application, issues such as persistence in the environment and non-target impacts will need to be researched thoroughly before EPA approval can be granted. Public acceptance may also be a hurdle, especially as the first variants of these RNAi control methods may find themselves in transgenic crops. Although RNAi-based insecticides will not be commercially available for several years, their many positive attributes in comparison to conventional insecticides hold the promise of revolutionizing arthropod pest control. Further information on the transcriptomes used to develop RNAi techniques for both species can be found in the following publications: Sparks ME, Shelby KS, Kuhar D, Gundersen-Rindal DE. Transcriptome of the Invasive Brown Marmorated Stink Bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Mittapalli O, ed. PLoS ONE. 2014;9(11):e111646. doi:10.1371/journal.pone.0111646. Sparks ME, Blackburn MB, Kuhar D, Gundersen-Rindal DE. Transcriptome of the Lymantria dispar (Gypsy Moth) Larval Midgut in Response to Infection by Bacillus thuringiensis. Terenius O, ed. PLoS ONE. 2013;8(5):e61190. doi:10.1371/journal.pone.0061190. Armando Rosario-Lebron: Is a graduate Student in the laboratory of Dr. Cerruti RR. Hooks at the University of Maryland that works on sustainable agriculture through cover crop management techniques. In the past he worked on genetic disorders in the eye in the laboratory of Dr. Jaqueline Tanaka at Temple University. Aditi Dubey is a PhD student in the Hamby Lab, studying non-target effects and sustainable use of neonicotinoid seed treatments. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290

 RSS Feed
RSS Feed




