|
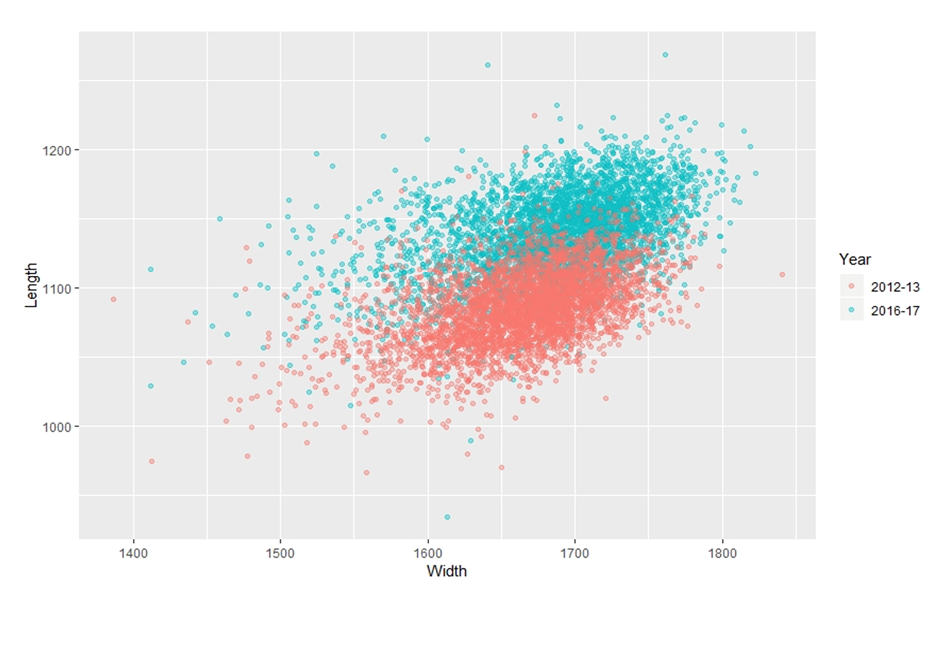
Written by: Lindsay Barranco Dr. Krisztina Christmon has measured approximately 100,000 mites during the course of her PhD studies at the University of Maryland, which may very well be a world-record. She recently presented her research exit seminar entitled “Varroa destructor: abiotic and biotic correlates to body size, and the effects of size and host type on mite tolerance to acaricide exposure” and described how her inquiry into mite size variability began in 2016, as a new PhD student in the UMD Bee Lab. The UMD Bee lab administers the National Honeybee Disease Survey (NHDS), and maintains collected data from large and small-scale beekeepers across the United States in order to detect honey bee disease and monitor for invasive species. Invasive species, particularly varroa mites, pose a serious threat to honey bee health which in turn, potentially impacts the important pollination services honey bees provide to fruit and vegetable growers nationwide. Varroa destructor is an obligatory ectoparasite of the honey bee, meaning that it is entirely dependent upon its host for its survival. The mite spends its entire life in the bee’s nest in immature phases or on adult bees where it is able to reproduce or travel in a phoretic phase to other hosts. Varroa destructor negatively impacts bees in two ways – by feeding on the fat body tissue of the honey bees and by vectoring numerous viruses, such as deformed wing virus. Varroa destructor began to spread from Asia to other areas worldwide in the 1950’s and is now a key challenge to beekeepers worldwide. An encounter with a smaller-than-usual mite specimen five years ago prompted Dr. Christmon to begin her research into varroa mite size differences and to determine what might contribute to mite size variability. She began her work by measuring NHDS mites from 2016-2017 and comparing those with stored NHDS mites from 2012-2013. In order to measure mites efficiently, Christmon developed protocols with the USDA Micoscopy department so that automatic measurement techniques could be used in measuring the length and width of each individual mite, resulting in high resolution imagery and allowing greater contrast for sizing analysis (Fig 1). Figure 1 (above): Varroa mites - raw images on the left needed to be converted into Fiji software for analysis and to provide greater contrast for sizing analysis (image on the right). Images by Dr. Krisztina Christmon. Dr. Christmon applied this novel measurement approach to understand drivers of mite size, as she evaluated numerous biotic and abiotic factors that could influence mite size variability between survey years 2012-2013 and 2016-2017. The five different abiotic correlates measured included year, month, season, State from which the mites were collected and pesticides. The four biotic correlates included varroa load, bee weight, virus load and genetics. Dr. Christmon found significant size variation within and between survey years 2012-2013 and 2016-2017 (Fig. 2). Findings also revealed that variation in the mean body width and length of mites was smallest in Fall and greatest in Spring, while the mean body width and length ratio was the greatest in Fall and Winter. Mean body width and length of mites was positively associated with exposure to the miticides coumaphos and thymol, as well as DWV-A (a variant of deformed wing virus). A negative association was found between the width, length, and the varroa load per 100 bees. Figure 2 (above). This graph (courtesy of Dr. Krisztina Christmon) depicts Varroa destructor size variability in the United States. Width measurements appear on the x-axis and length measurements appear on the y-axis. Green dots are from survey year 2016-2017, and red dots are from survey year 2012-2013. This graph indicates that there is great size variability within the survey years and between the survey years.
As part of her work, Dr. Christmon also examined how long-term storage of mites in ethanol alcohol might impact mite size. She selected mites randomly from a local Maryland colony and repeatedly took images of the mites over 90 weeks. Christmon found that the difference in mite size between the survey years is largely due to storage in ethanol. As she continued the descriptive study of Varroa destructor size variability, Christmon found an association between apiary-level acaricide and mite size, leading to a survey of amitraz-resistance in the U.S mite population and the role of mite size in survival. To more deeply understand the relationship between body size and miticide resistance, Dr. Christmon collaborated with the Bee Informed Partnership, which conducted Michigan field trials on two types of acaricides – amitraz and thymol. The controlled experimental trials were established in three different locations, with 12 colonies per site. Five sampling events occurred over a 42 day period, with a total of 36 colonies sampled over the duration of the trials. Colonies were purposefully exposed to either amitraz or thymol. Christmon measured varroa mites collected from these colonies and analyzed the impact of body size on mite mortality. Her findings suggest that amitraz kills smaller mites but is not as effective at killing larger mites. Christmon conducted similar work using Maryland honey bee colonies and confirmed that larger mites had a higher survival rate than mites that were medium or smaller in size. Altogether, these studies indicated that smaller mites are more susceptible to pesticides in toxicological bioassays than larger mites. While further research is needed, these findings suggest important implications for beekeepers in the control of Varroa destructor and that miticide choice, application and timing of those applications are key to effective varroa control. Lindsay Barranco is a Master student in the UMD Bee Lab studying solitary bee nesting preference in varied soil substrates. References: Hernández-Rodríguez, Carmen Sara, et al. "Resistance to amitraz in the parasitic honey bee mite Varroa destructor is associated with mutations in the β-adrenergic-like octopamine receptor." Journal of Pest Science (2021): 1-17. Hasegawa, Nonno, Maeva A. Techer, Noureddine Adjlane, Muntasser Sabah al-Hissnawi, Karina Antunez, Alexis Beaurepaire, Krisztina Christmon et al. "Evolutionarily diverse origins of honey bee deformed wing viruses." bioRxiv (2023): 2023-01. Rosenkranz, Peter, Pia Aumeier, and Bettina Ziegelmann. "Biology and control of Varroa destructor." Journal of invertebrate pathology 103 (2010): S96-S119. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290




 RSS Feed
RSS Feed




