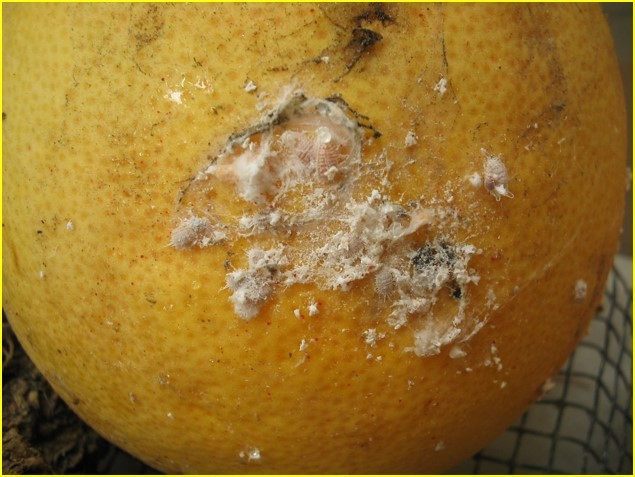
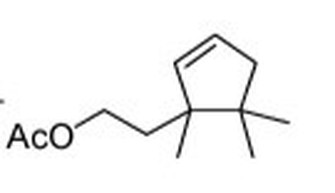
Sexy-smelling compounds provide a promising tool for mealybug management Mealybugs (family Pseudococcidae) are small insect pests commonly an issue in greenhouses and nurseries, especially in warmer climates. Dr. Rebeccah Waterworth, a postdoc in the University of Maryland’s entomology department, did her dissertation work while at the University of California Riverside on mealybugs in a Californian production nursery setting. Mealybugs are among the least apparent of pests. They are small insects and are only easy to see once they have accumulated in large numbers (Figure 1). On top of that, current monitoring methods for mealybugs often involve destructive sampling of plant material, which is problematic if a nursery is trying to produce blemish-free plants. If one could detect them earlier, more options would be available to get rid of them before they start damaging the plants. Figure1: Accumulation of obscure mealybugs. Photo credit Rebeccah Waterworth. Sex pheromones have been successful in other cropping systems (e.g. as a mating disruption method in moths in cranberry, Deutsch et al. 2014); however using sex pheromones as a monitoring technique of mealybugs is relatively recent. The chemistry is ready: sex pheromones of some species of mealybugs have already been isolated in a laboratory setting. By placing sticky traps with a lure saturated in the female sex pheromone out in the field, one can collect the male mealybugs (Figure 2) attracted to the pheromone. The presence of male mealybugs implies that there is in fact a mealybug population present. But could those traps be used to detect, monitor and estimate mealybug population size in a nursery? Figure 2: A mealybug pheromone trap. Photo credit Rebeccah Waterworth. Rebeccah set out to answer these questions in ornamental nurseries in California (Waterworth et al. 2011a). By placing sticky traps out in the field with lures of different pheromone doses she found that the male mealybugs are sensitive to very low doses of female sex pheromone. This is good news, because the mealybug pheromone chemicals (Figure 3) are complex molecules that are difficult and expensive to make in a laboratory setting, so the less one needs, the better. Additionally, she used the pheromone traps to estimate mealybug abundance. First, traps were set out, mealybug males were collected and counted and then compared to the true density of mealybugs found in plant samples. This resulted in a mathematical relationship that can be used to estimate true mealybug abundances in the field. Pheromone based sticky traps can now be utilized by growers to monitor mealybug populations. Figure 3: Structure of the longtailed mealybug mating pheromone. Another potential use for pheromones comes in the form of mating disruption. If enough pheromones are released at once, the male insects become inundated with signals and are unable to follow the trail left by an actual female. Without this path to the female, no mating occurs. Rebecca wanted to determine if mating disruption was a feasible tool for combating mealybugs. In testing this feasibility, Rebeccah learned some interesting aspects of mealybug biology (Waterworth et al. 2011b). First, the three species of female mealybugs she studied are not parthenogenetic, so they have to mate with a male to produce offspring. This was good news: if pheromones could prevent the males from getting to the females, there would be no future generations of mealybugs. Second, female mealybugs live for a very long time for an insect. The longtailed mealybug female, for example, lived up to 137 days in lab trials. Unfortunately this news was less welcome. Because of their long lifespans, pheromone clouds would need to be running continuously in nurseries for over 3 months to keep the males at bay. Although possible, keeping a control system running for that length of time would require a large quantity of synthetic pheromone. Currently, pheromone synthesis is such a complex process that only 5g of pheromone can be produced at a time, which increases costs. Rebeccah’s message was that if this process is simplified in the future, mating disruption could become a feasible control method. In all, Rebeccah’s talk showcased not only the potential uses of pheromone trapping and mating disruption in a new pest system, but also the long process that is necessary for the development of these tools. Future practical applications of these mating pheromones could not be assessed without the comprehensive understanding of mealybug reproductive biology she gained through her research. References:
Deutsch, A., A. Mafra-Neto, J. Sojka, T. Dittl, J. Zalapa, and S. Steffan. 2014. Pheromone-based mating disruption in Wisconsin cranberries. Wisconsin State Cranberry Growers Association. Waterworth, R. A., R. A. Redak, and J. G. Millar. 2011a. Pheromone-baited traps for assessment of seasonal activity and population densities of mealybug species (Hemiptera: Pseudococcidae) in nurseries producing ornamental plants. J. Econ. Entomol. 104: 555-565. Waterworth, R. A. I. M. Wright, and J. G. Millar. 2011b. Reproductive Biology of Three Cosmopolitan Mealybug (Hemiptera: Pseudococcidae) Species, Pseudococcus longispinus, Pseudococcus viburni, and Planococcus ficus. Ann. Entomol. Soc. Am. 104: 249-260. About the Authors: Olivia Bernauer is a first year Master’s student in Dennis vanEngelsdorp’s bee lab working with wild, native bees. Olivia is currently working with volunteers to monitor the diversity and floral preference of Maryland’s native bees. Becca Wilson is a third year PhD student in Bill Lamp’s lab. She is studying the distribution patterns and societal impact of nuisance black flies in western Maryland. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290


 RSS Feed
RSS Feed




