 The kissing bug, Rhodnius prolixus. © Jena Johnson The kissing bug, Rhodnius prolixus. © Jena Johnson Written by: Arielle Arsenault-Benoit & Katie Reding Vector development and reproduction are imperative to combating vector-borne illness. Dr. Kevin Vogel and his research team at the University of Georgia are employing an integrative approach to further understanding of these factors in mosquitoes and a triatomine kissing bug. 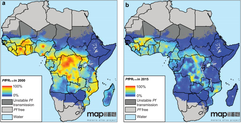 Figure 1. Reduced incidence of the malaria parasite, Plasmodium falciparum from 2000 (a) to 2015 (b). From Bhatt et al. 2015 Figure 1. Reduced incidence of the malaria parasite, Plasmodium falciparum from 2000 (a) to 2015 (b). From Bhatt et al. 2015 Interventions againts vector-borne illness using traditional pest management strategies have been quite successful, as demonstrated by the reduced incidence of malaria in Africa over time (Fig 1, Bhatt et al, 2015). Yet, the threat of evolved resistance to insecticde treatments has prompted researchers to study basic biology of insect vectors such as mosquitos and kissing bugs. Dr. Kevin Vogel, Assistant Professor at University of Georgia, pursues a greater understanding of vector physiology and their microbial symbionts to inform disease control efforts. Dr. Vogel devoted the first half of the seminar to his work identifying the receptor of ovary ecdysteroidogenic hormone in the mosquito Aedes aegypti. In A. aegypti, like all mosquitoes, females require a blood meal prior to oocyte (egg) maturation and subsequent egg laying. Once a female mosquito has obtained a blood meal, peptide hormones produced in the brain are transported to the ovaries where they initiate a signaling cascade that results in the secretion of yolk proteins from the fat body to the oocytes (a process known as vitellogenesis, after the yolk protein vitellogenin). There are two peptide hormones in A. aegypti known to transmit this signal from the brain to the ovaries: ovary ecdysteroidogenic hormone (OEH) and insulin-like peptide 3 (ILP3). While the receptor of ILP3 is known to be the mosquito insulin receptor (MIR), the OEH receptor had been a mystery. 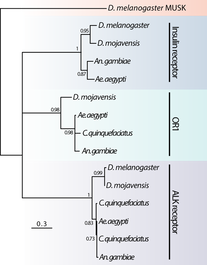 Figure 2. AAEL001915 is the only member of the OR1 clade. Note that a D. melanogaster ortholog was not found. Figure 2. AAEL001915 is the only member of the OR1 clade. Note that a D. melanogaster ortholog was not found. Jan Veenstra showed that none of the species in the melogaster subgroup of Drosophila have a gene encoding OEH (2010). Based on the observation that loss of a ligand is generally coincident with loss of the ligand’s receptor, Dr. Vogel hypothesized that whatever protein is the receptor of OEH in A. aegypti would probably not be found in the genomes of these Drosophila species. To identify the OEH receptor, Dr. Vogel and colleagues narrowed down their search with the following criteria: 1) the receptor should be one of the types that is known to bind peptide hormones (G protein-coupled receptors, protein receptor kinases, and receptor guanylyl cyclases), 2) it should be found in most dipteran genomes except genomes of species in the melanogaster subgroup of Drosophila, and 3) it should be an orphan receptor (a receptor for which the ligand is unknown). Discovering which proteins meet all these specifications required a fine-scale phylogeny of dipteran peptide hormone receptors, so Dr. Vogel first mined five Dipteran genomes for sequence of these receptors (Vogel et al., 2013). Once their phylogenetic tree was complete, the group found only one peptide hormone receptor was a good candidate given their hypotheses: AAEL001915 (Figure 2). 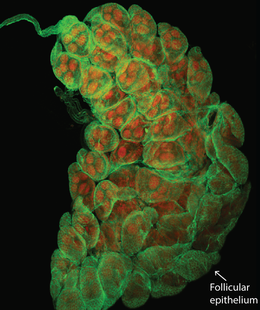 Figure 3. A. aegypti ovaries stained for AAEL001915 (green) and nuclei (red). From Vogel et al., 2015 Figure 3. A. aegypti ovaries stained for AAEL001915 (green) and nuclei (red). From Vogel et al., 2015 To test whether AAEL001915 was involved in vitellogenesis, Dr. Vogel et al. knocked down the gene’s function by RNA interference (RNAi) and subsequently measured yolk deposition in females after being fed a blood meal. AAEL001915 RNAi females deposited less than a quarter the amount of yolk than was found in control females’ oocytes. Using an antibody that binds AAEL001915, they localized AAEL001915 to the follicular epithelium of the A. aegypti ovaries (Fig. 3). Furthermore, they showed that the reduction in egg deposition in RNAi-treated females could not be rescued even when exogenous OEH was supplied to the knockdown ovaries. This result is consistent with AAEL001915 being the OEH receptor; presumably if some other protein were the OEH receptor, this assay would not affect its function, and it would be able to bind the exogenous OEH, initiating the process of vitellogenesis and allowing yolk deposition in oocytes to proceed normally. They then showed by immunoblotting that AAEL001915 forms a dimer and that an antibody that binds OEH also pulls down a protein that is the same size as the AAEL001915 homodimer. Using all these pieces of evidence, among others (see Vogel et al., 2015 for more details), Dr. Vogel and colleagues concluded that AAEL001915 is indeed the mysterious OEH receptor, so both ligand and receptor are orphaned no longer. 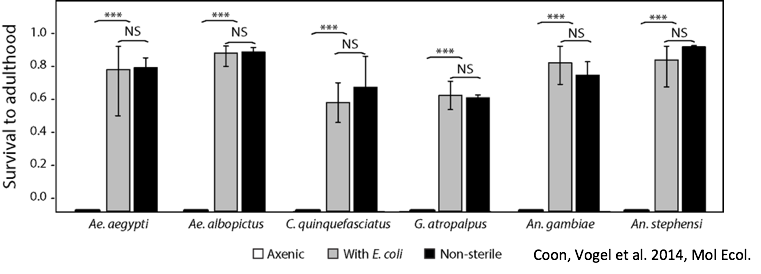 Figure 4. Axenic larvae do not survive to adulthood, but they do survive both in non-sterile media and when rescued with E. coli. This process is conserved across multiple species. From Coon et al. 2014. Figure 4. Axenic larvae do not survive to adulthood, but they do survive both in non-sterile media and when rescued with E. coli. This process is conserved across multiple species. From Coon et al. 2014. For the remainder of the seminar, Dr. Vogel shifted his focus to a discussion of the role of bacterial symbionts in the development of insect vectors of disease. In their juvenile life stages, mosquitoes are restricted to aquatic environments, where they gain the energetic reserves available to them when they emerge as adults. This portion of the life cycle, though imperative for mosquito development, does not garner as much research as does the adult stage. Dr. Vogel and his colleagues found that axenic larvae- those that are not exposed to microbes- will not molt from the first larval instar stage to the second larval instar stage (Figure 4). Yet, this process of development can be rescued with the addition of non-sterile media containing bacteria. Even the larvae of Toxorhynchites spp., mosquitoes that are predatory in their larval stage, and therefore have access to non-microbial nutrition, require bacteria to develop. Therefore, there must be some aspect of the microorganisms associated with mosquitoes in their larval stage that is required for development. Many species of bacteria can facilitate this process, and the bacteria found in larvae are identical, although in different relative abundance, to those in the aquatic environment. 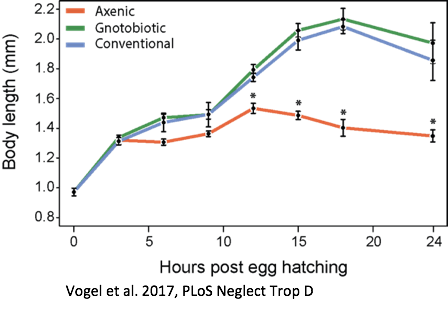 Figure 5. Axenic larvae do not reach the critical size to molt to the second instar, but mosquitoes exposed to conventional media or a single bacteria (gnotobiotic) Figure 5. Axenic larvae do not reach the critical size to molt to the second instar, but mosquitoes exposed to conventional media or a single bacteria (gnotobiotic) Dr. Vogel and his research team explored this relationship through a series of experiments. Heat-killed bacteria were fed to larvae, which did not rescue the development process. They found that bacteria needed to be alive to facilitate the process, but many species of bacteria can serve the purpose. Dr. Vogel and his colleague Dr. Kerri Coon found that bacteria within the mosquito gut consume oxygen. Because many bacteria species can serve this purpose, researchers were able to use Escherichia coli, a well-studied bacteria that can be manipulated in the laboratory. The oxidative respiration gene was “knocked out”, or removed, from E. coli, which was then fed to otherwise sterile mosquito larvae. The larvae consumed the modified bacteria, but the molting process did not proceed, suggesting that the bacteria within the gut had to be consuming oxygen to prompt the molting process. These axenic larvae still consumed food at the same rate as non-sterile larvae and were able to survive in this state, but never reached the size required to molt into the subsequent instar (Figure 5). Through an RNASeq experiment, Dr. Vogel saw a significant reduction in digestive peptides in axenic larvae, as well as a reduced ability to absorb nutrients. The results demonstrated an increase in the use of metabolic pathways consistent with starvation.  Figure 6. The kissing bug, Rhodnius prolixus. © Jena Johnson Figure 6. The kissing bug, Rhodnius prolixus. © Jena Johnson The Vogel lab is now in the early stages of exploring a similar relationship between bacteria and an additional insect vector, Rhodnius prolixus. Rhodnius prolixus (Figure 6) is a triatomine kissing bug, and the primary vector of parasite that causes Chagas Disease, Trypanosoma cruzi. Like mosquitoes, kissing bugs require a bloodmeal from a vertebrate host, which makes them particularly proficient at vectoring disease. The kissing bug has an obligate relationship with the bacteria Rhodococcus rhodinii in its gut. This microbe is likely essential for the survival of R. prolixus, and Dr. Vogel’s research team is exploring which genes are involved in this association. Like E. coli, R. rhodinii can be cultured and transformed in the laboratory, which makes genetic experiments possible. Genes can be knocked out, and these modified bacteria can be artificially fed to the bugs to determine if each gene is critical to the process. The research of Dr. Vogel and his laboratory members offer insights into the physiology and host-microbe interactions of insect vectors that are of particular importance to the Americas. You can find more about his lab at the University of Georgia here. References Bhatt, Samir, D. J. Weiss, E. Cameron, D. Bisanzio, B. Mappin, U. Dalrymple, K. E. Battle et al. (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature, 526 (7572). Coon, K.L., Vogel, K.J., Brown, M.R. and Strand, M.R., (2014). Mosquitoes rely on their gut microbiota for development. Molecular ecology, 23(11), 2727-2739. Veenstra, J. (2010) What the loss of the hormone neuroparsin in the melanogaster subgroup of Drosophila can tell us about its function. Insect Biochemistry and Molecular Biology, 40(4). Vogel, K., Brown, M., & Strand, M. (2013) Phylogenetic investigation of peptide hormone and growth factor receptors in five dipteran genomes. Endocrinol., 4(193). Vogel, K., Brown, M., & Strand, M. (2015) Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. PNAS, 112(16). Vogel, K.J., Valzania, L., Coon, K.L., Brown, M.R. and Strand, M.R., (2017) Transcriptome sequencing reveals large-scale changes in axenic Aedes aegypti larvae. PLoS Neglected Tropical Diseases, 11(1). About the authors Arielle Arsenault-Benoit is a first-year PhD student in the Fritz lab who studies ecology and landscape genomics of Culex pipiens mosquitoes on an urban to rural gradient. Katie Reding is a first-year PhD student in the Pick lab who studies the formation of segments in the embryo of a hemipteran. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290

 RSS Feed
RSS Feed




