[Seminar Blog] Mitey talk: Dr. Zachary Lamas' exit seminar on honey bee's disease transmission4/19/2022
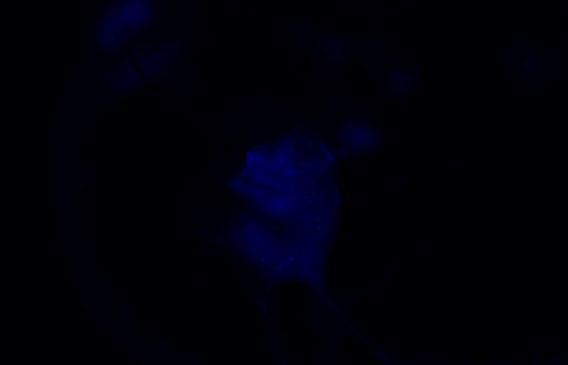
His story began 10 years ago, when Dr. Lamas had an epiphany at the top of his ladder while fixing a roof: “Mom, I want to go to grad school. Would you help me with that please?” now, 10 years later, his parents are holding hands, their eyes are full of pride and happiness listening to their son share his remarkable story with us. With a background in beekeeping and prior experience running a successful Queen breeding business, Dr. Lamas set out to solve a number of fundamental questions pertaining to the honeybee parasitic mite: Varroa destructor. V. destructor, is the most important driver of honeybee colony losses worldwide. Mites damage bees in two ways; directly by feeding on honeybee pupae and adult bees and indirectly by vectoring bee viruses like the deformed wing virus (DWV). DWV is a highly important disease agent for honeybees worldwide. This virus is especially damaging when co-occurring with the parasitic mite Varroa destructor, a known vector [1-4]. In fact, while DWV circulates in most honeybee colonies, this virus is rarely symptomatic unless vectored by Varroa mites [5-9].Despite being so detrimental to the industry, questions surrounding the mite’s basic biology remain unanswered. These questions became the focus of the first part of Dr. Lamas’ PhD career. 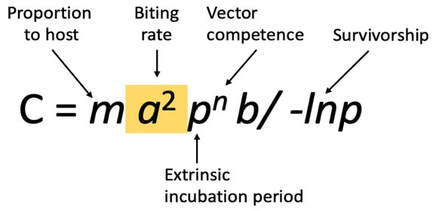 Figure 1 is the original vectorial capacity equation described in 1964 by Garret-Jones. The biting rate is highlighted. Figure 1 is the original vectorial capacity equation described in 1964 by Garret-Jones. The biting rate is highlighted. The first two questions of particular interest to Dr. Lamas were:1) Do mites move from one bee to another to feed? And 2) What is the biting rate of an arthropod vector, and why does it matter in Varroa-honeybee relationships? To investigate these questions, Dr. Lamas applied Garret Jones’ (1964) bacterial capacity model [10] to understand varroa host switching (Figure 1). This vector centric model is used to describe the number of infectious individuals that would arise from one infectious vector in a day. Of all the parameters in the model, the biting rate overwhelmingly shapes disease transmission [11]. Using this equation in combination with a lab experiment monitoring mite movement on painted bees within many honeybee microcolonies, Dr. Lamas showed that these mites do move from one bee to another, and that a small increase in biting rate has a non-linear impact on the number of infected individuals. Another interesting, intertwining question of interest to Dr. Lamas was: are mites actually feeding when in the assumed feeding position? To address this question, Zac developed a way to inject fluorescent microspheres into adult bees and then use these microbeads to visualize feeding by examining mites removed from treated bees for the presence of the fluorescent microbeads. Using this method, Zac was able to confirm that mites specifically enter this space on the honeybee body to feed (Figure 2). 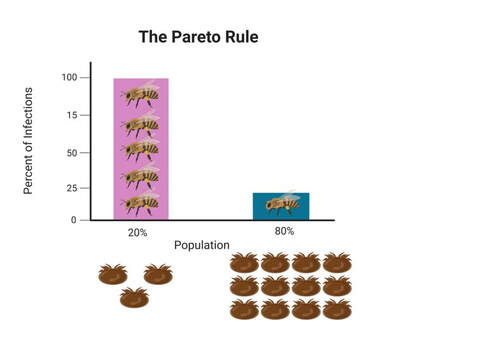 Figure 3 visualizes the application of the Pareto Rule to honeybee-varroa disease transmission. Figure 3 visualizes the application of the Pareto Rule to honeybee-varroa disease transmission. So why does this matter? Previous research investigating host switching in mosquitoes and ticks has shown that the highest frequency biters are responsible for the most disease transmission, resulting in the highest number of parasitized hosts. Called the Pareto Rule, this generalization, when applied to disease vectors, states that around 20% of vectors (those with the highest biting frequency) are responsible for the majority (roughly 80%) of disease transmission and parasitized individuals [11]. This finding was confirmed through an additional aspect of Dr. Lamas’ research (Figure 3). Host switching rate, confirmation of feeding position, and the application the Pareto Rule to Varroa-honeybee relationships all comprise just a small aspect of the vast amount of research performed during Dr. Lamas’ impressive PhD career. Keep an eye out for future publications by Dr. Zachary Siqueira Lamas to learn more about his honeybee research! Authors: Krisztina Christmon, PhD student at vanEngelsdorp bee lab studying the honey bee parasite: Varroa destructor – size, feed state and its association with survival of acaricide exposure. Veronica Yurchak, PhD student in Dr. Cerruti Hooks’ applied ecology lab studying alternative methods for pest control and natural enemy enhancement in vegetable production. References 1. Steinhauer, N., et al., Drivers of colony losses. Current Opinion in Insect Science, 2018. 26: p. 142-148. 2. Ryabov, E.V., et al., A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis mellifera) Prevails after Varroa destructor-Mediated, or In Vitro, Transmission. PLOS Pathogens, 2014. 10(6): p. e1004230. 3. Ryabov, E.V., et al., Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Scientific Reports, 2017. 7(1): p. 17447. 4. Ryabov, E.V., et al., Dynamic evolution in the key honey bee pathogen Deformed wing virus. bioRxiv, 2019: p. 653543. 5. Kang, Y., et al., Disease dynamics of honeybees with Varroa destructor as parasite and virus vector. Mathematical Biosciences, 2016. 275: p. 71-92. 6. Evans, J.D. and R.S. Schwarz, Bees brought to their knees: microbes affecting honey bee health. Trends in microbiology, 2011. 19(12): p. 614-620. 7. Wilfert, L., et al., Deformed wing virus is a recent global epidemic in honeybees driven by <em>Varroa</em> mites. Science, 2016. 351(6273): p. 594-597. 8. Di Prisco, G., et al., Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proceedings of the National Academy of Sciences, 2013. 110(46): p. 18466-18471. 9. Di Prisco, G., et al., Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. Journal of General Virology, 2011. 92(1): p. 151-155. 10. Garrett-Jones, C., Prognosis for Interruption of Malaria Transmission Through Assessment of the Mosquito's Vectorial Capacity. Nature, 1964. 204(4964): p. 1173-1175. 11. Cooper, L., et al., Pareto rules for malaria super-spreaders and super-spreading. Nature Communications, 2019. 10(1): p. 3939. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290



 RSS Feed
RSS Feed




