|
writen by: Demian A. Nuñez and Darsy Smith The end of the semester is here! For the last weekly seminar series, the Entomology Department welcomed Dr. Jason Rasgon to speak about his research and experience with new molecular tools for gene editing in arthropods. Dr. Rasgon completed his Ph.D. at the Entomology Department of the University of California, Davis. There he had the opportunity to conduct research on Wolbachia infection dynamics, a naturally occurring bacteria that lives within several insect taxa and is passed from one generation to the next through their eggs. Since early in his career, he has successfully answered research questions in various systems, which has given him the experience to lead projects across a wide range of biology-subdisciplines. Currently, he is a professor of Entomology and Disease Epidemiology at Pennsylvania State University, where he integrates population biology, ecology, molecular tools, and theory to answer both fundamental and applied questions in genetics. The main goal of Dr. Rasgon’s lab is the development of new methods that can be used to introduce transgenes into natural disease vector populations.
Embryonic injection also comes with the drawback of not being applicable to many species because of potential damage to target eggs, or the reproductive physiology of target species making the technique impossible or impractical.
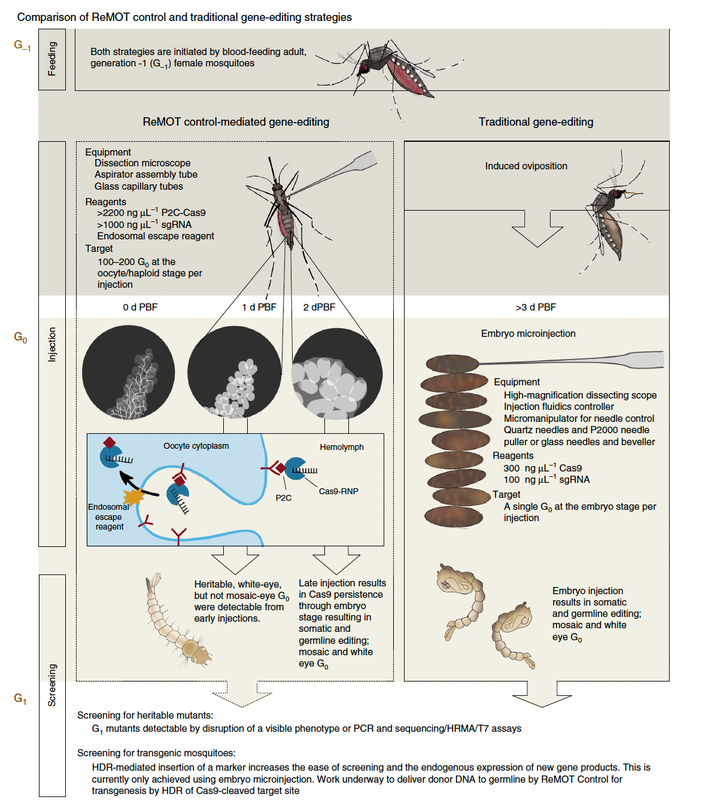
The aforementioned issues dramatically limit the use of CRISPR-Cas-9 technology in many systems and research settings. ReMOT Control, in contrast, is easy to employ and is applicable to a wide variety of oviparous species, making it incredibly useful for delivering Cas-9 and enacting heritable germline modification in many different arthropod lineages. ReMOT Control is based on the principle that most egg-laying animals deliver protein to eggs developing in their ovaries through a process called vitellogenesis, which is conserved across most oviparous species. In arthropods, yolk protein precursors (YPP) secreted by the fat body are taken up by the ovaries through a process called receptor-mediated endocytosis (RME). During this process, receptors on the oocyte membrane bind with YPP and are brought into the cells. Using ligands derived from YPP, it is possible to deliver macromolecules such as Cas9 ribonucleoprotein (RNP) and Cas-9 single-guide RNA (sgRNA) into the eggs of vitellogenic female insects simply by injecting them into the hemolymph. The process has already been successfully demonstrated across many species, including Aedes aegypti, which is a significant vector for many human diseases such as malaria. The success of this technique opens the door for more affordable and accessible research into genetic technology around the world. Such developments may include gene drives that could reduce the ability of disease vectors to transmit human pathogens or possibly reintroduce vulnerability to pesticides to certain insect populations. As a tool, ReMOT Control has incredible potential to revolutionize the way genetic engineering research is done in the 21st century. References Chaverra-Rodriguez, D., Macias, V. M., Hughes, G. L., Pujhari, S., Suzuki, Y., Peterson, D. R., ... & Rasgon, J. L. (2018). Targeted delivery of CRISPR-Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nature communications, 9(1), 1-11. Authors Demian A. Nuñez is an MS student studying the potential biocontrol benefits of intercropping perennial ground cover with cucurbit crops as an alternative to traditional chemical control strategies. Darsy Smith is a PhD student in the Lamp lab studying host plant resistance and response to natural enemies in alfalfa ecosystems. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290

 RSS Feed
RSS Feed




