[Seminar blog] The end of spray and pray? Alternative ways to control spotted wing drosophila4/25/2022
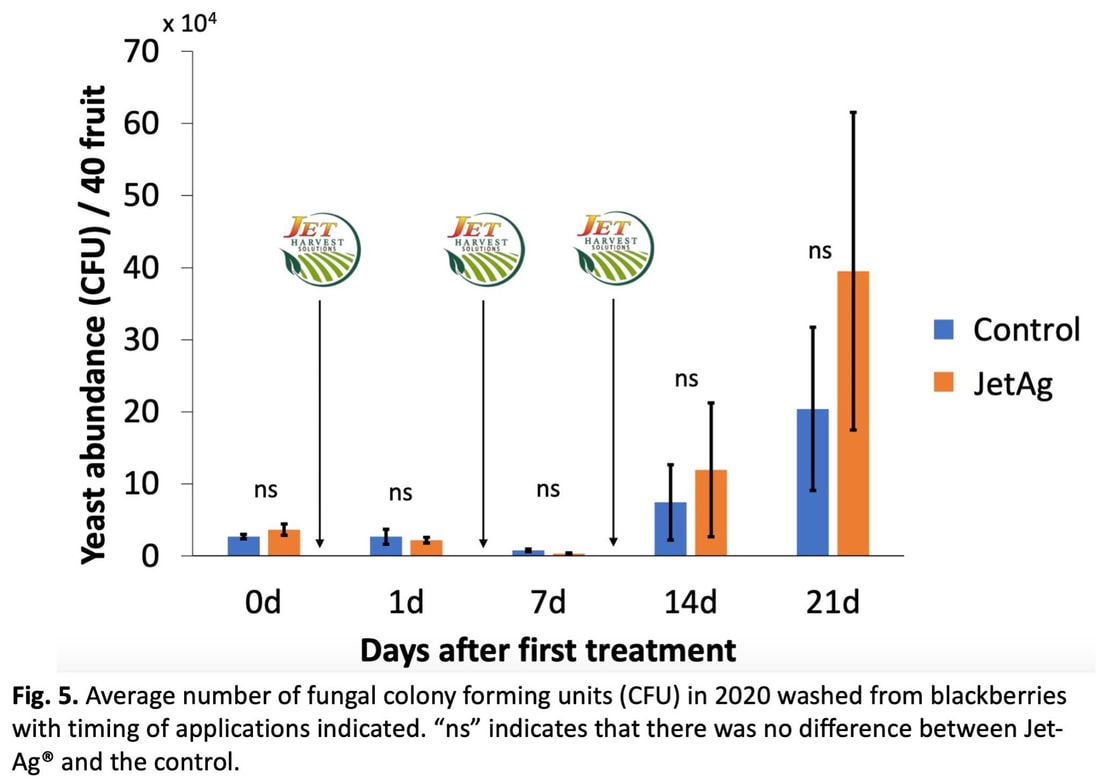
 written by: Maria Cramer and Huiyu Sheng Spotted wing drosophila (SWD) is a pest that causes backyard berry growers and commercial farmers alike a lot of grief. Native to Eastern Asia, this fruit fly (closely related to the famous Drosophila melanogaster) first appeared in the mainland U.S. in 2008. Like other fruit flies, SWD lays eggs in fruit, but unlike others, it has a sharp ovipositor that allows it to cut through fruit skin and lay eggs inside (Figure 1). Because of this, SWD larvae aren’t restricted to rotting or damaged fruit– they can infest otherwise perfect produce. Their favorite fruits include raspberries, blackberries, grapes, and cherries. Besides the ick-factor, the wounds from cutting into fruit can introduce microorganisms that cause the fruit to rot. To make things worse, because they’re inside fruit, the larvae are protected from insecticide sprays (Figure 2). This means farmers have to instead target the adult flies, which requires frequent insecticide applications. Spraying insecticides over and over has many downsides; it’s expensive for farmers, it could hurt other insects, and it could even put pressure on SWD to develop resistance to the chemicals themselves, making them less effective. Dr. Torsten Schöneberg would love to see an end to this “spray and pray” approach for managing SWD. Currently a researcher at Agroscope in Switzerland, Dr. Schöneberg recently completed a postdoc in the Hamby Lab at the University of Maryland. He returned to the Entomology Department’s weekly seminar series to give an update on the research he did during this time. 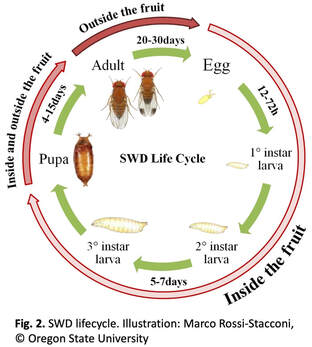 Dr. Schöneberg explored two different tactics for trying to reduce SWD in blackberries and raspberries (caneberries) without using insecticides. Both methods relied on making the plants less hospitable, so SWD would be less likely to lay eggs there. The first approach took advantage of SWD temperature and humidity preferences: SWD prefers cooler, more moist environments (Schöneberg, et al, 2021), and tends to preferentially infest fruit in the middle of dense foliage. By removing some canes, or using a trellis to spread canes apart, there could be less shade and moisture inside the plants, which might decrease damage. To test whether manipulating blackberry growth might change their microclimate and reduce SWD infestation, Dr. Schöneberg compared two trellises. The standard trellis in his study created a single row of canes, held up by twine wrapped around single stakes– this is the type of trellis that most farmers use in Maryland, and you might see it if you go to a pick-your-own farm. In contrast, the experimental trellis stakes were shaped more like a “T” and allowed the canes to be spread apart in V-shape (Figure 3), creating an open center. Dr. Schöneberg then measured the temperature and humidity to see if the microclimate was really being affected. When the fruit ripened, he harvested weekly, divided it into marketable and unmarketable (using a “would you buy it?” test), measured sugar content and firmness, then counted how many larvae were infesting the fruit using a sugar-flotation method. However, after taking all of these measurements, it turned out that there were no differences between the trellises– the fruit was similar quality, had similar infestation, and there weren’t significant temperature changes. While these results could have just been disappointing, they were also interesting. There was a clear relationship between temperature and amount of infestation – the warmer the temperature, the lower the infestation. This means that if a different type of trellis, or in a different growing area, it might be possible to modify the plants to reduce infestation, even if it didn’t work in this case. The second approach Dr. Schöneberg tried was using Jet-Ag® to control SWD in blackberries. Jet-Ag® (mix of peroxyacetic acid & hydrogen peroxide) is an organic crop sanitizer used by growers against fungi, including yeasts and pathogens. Yeast occurs naturally on fruits and plays an essential role in SWD egg laying and larval growth (Anagnostou, et al, 2010), and a variety of yeast genera are associated with both larvae and adult SWD (Lewis, et al, 2019). Dr. Schöneberg wondered whether Jet-Ag® could reduce these important SWD-associated yeasts and thus make fruit less hospitable to SWD. Dr. Schöneberg first performed an in vitro (in the lab environment) study testing if Jet-Ag® is really able to affect yeast growth. He performed an agar plug diffusion assay by culturing yeast on agar plates with a hole in the middle for one hour or 24 hrs. Then, he injected different concentrations of the Jet-Ag® solution into the hole and observed the yeast colony growth. Dr. Schöneberg found that yeast growth decreased as the concentration of Jet-Ag® increased, indicating Jet-Ag® is able to inhibit yeast in the lab environment (Figure 4). To test whether Jet-Ag® reduced yeast growth on fruit, a student in the Hamby lab dipped store-bought, surface sterilized blackberries in a yeast suspension, followed by the Jet-Ag® solution (or water as a control). After the blackberries dried, the student washed them, and the wash water was diluted and plated on agar plates. Several days later, colonies on the agar plates were measured in colony forming units (CFUs). Consistent with the result of the agar plug diffusion assay, more CFUs were observed in the water-treated group than Jet-Ag®-treated group. This suggested Jet-Ag® prevents yeast growth and causes contact mortality to yeast on the fruit, at least in the lab.
As for the central question of this talk, is it the “end of spray and pray?”, the short answer is: No! Dr. Schöneberg mentioned we still need to develop non-insecticidal tactics to control SWD population. Depending on the crops and their location, we need to combine direct and indirect measures.
References: Schöneberg T, English LA, Popp J, Hamby KA. Impact of Modified Caneberry Trellis Systems on Microclimate and Habitat Suitability for Drosophila suzukii (Diptera: Drosophilidae), Journal of Economic Entomology, 2021; https://doi.org/10.1093/jee/toab236 Schöneberg T, Lewis MT, Burrack HJ, Grieshop M, Isaacs R, Rendon D, Rogers M, Rothwell N, Sial AA, Walton VM, Hamby KA. Cultural Control of Drosophila suzukii in Small Fruit—Current and Pending Tactics in the U.S. Insects. 2021; https://doi.org/10.3390/insects12020172 Anagnostou C, Dorsch M, Rohlfs M. (2010), Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomologia Experimentalis et Applicata, 136: 1-11. https://doi.org/10.1111/j.1570-7458.2010.00997.x Lewis MT, Hamby KA. Differential Impacts of Yeasts on Feeding Behavior and Development in Larval Drosophila suzukii (Diptera:Drosophilidae). Sci Rep 9, 13370 (2019). https://doi.org/10.1038/s41598-019-48863-1 Author Bios: Maria Cramer is a PhD student in the Hamby lab studying sustainable pest control and non-target effects of insecticides in field corn. Huiyu Sheng is a PhD student in the St. Leger lab studying genomic and phenotypic differences among Metarhizium robertsii strains. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290



 RSS Feed
RSS Feed




