Spotted wing drosophila-microbe interactions: Integrating ecological complexity into SWD management4/22/2021
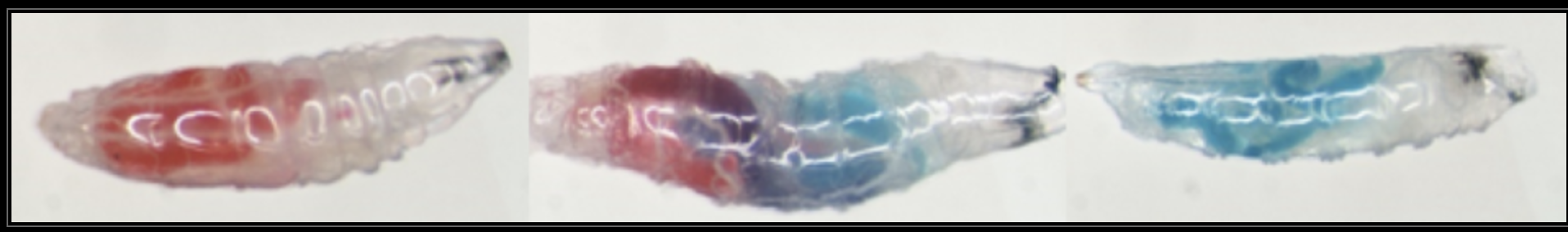
written by: Huiyu Sheng and Arielle Arsenault-Benoit Maggie Lewis, a PhD student in the Hamby Lab, aims to advance sustainable management of spotted-wing drosophila (Drosophila suzukii, SWD). Spotted-wing drosophila is a pest of soft skinned fruits such as raspberries and blackberries that is specifically adapted to infest ripe fruit prior to harvest, unlike other fruit flies that often lay their eggs in overripe or rotting fruit. Spotted Wing Drosophila presents a considerable threat to the small fruits industry because the management options are limited and the consumer tolerance threshold to larvae in fruit is very low. Lewis’s dissertation work aims to inform and improve traditional Integrated Pest Management (IPM) approaches for SWD through a better understanding of the fly’s relationships with yeasts, synergism with fruit rot fungi, and optimization of traditional management practices. Yeast plays a variety of roles in nature and is considered an important source of dietary lipids and proteins for many Drosophila species, including SWD. Within fruit, SWD encounters and feeds upon a diverse community of yeast microbes. However, previous surveys suggest that SWD may associate with one species of yeast in particular, Hanseniaspora uvarum. In previous surveys of larval microbial associations, over 90% of SWD larvae sampled in MD and CA were found to have fed on HU [1]. In order to determine if this predominance of HU reflects the field density or is caused by larval feeding preference, Lewis evaluated the larval preference of several naturally associated yeast species with Saccharomyces cerevisiae (SC) as a positive control. Although SC is not naturally associated with field populations of Drosophila, it is commonly used as a model organism for laboratory studies of Drosophila – yeast interactions. Lewis provided each SWD larva with two choices of yeast on each side of the arena with each yeast species dyed in either red or blue (Figure 2). She recorded the gut color of each larva after one hour and found that HU is significantly preferred by SWD larvae compared to the other yeast species [2]. Additionally, Lewis set up a larval development assay to evaluate the effect of different yeast species on SWD fitness and development. She reared SWD larvae on different yeast cultures and recorded the pupation, eclosion (adult emergence) time, and the adult body size for each individual. She found that larvae that lived on HU had a higher eclosion rate and a shorter eclosion time compared to larvae on other yeast species except for SC [2]. Surprisingly, there is a mismatch between larval yeast preference and performance. Fly body size provides a measure of overall adult robustness and fitness. Flies that were reared on the SC plate are the largest flies, whereas those that were reared on HU plate turned out to be the smallest among the four yeast species, even though HU was the preferred food, suggesting that factors beyond yeast nutritional quality may drive larval feeding behavior [2]. Flies' interactions with microbes go far beyond simply feeding [3]. Lewis also found that adult SWD carry a diverse community of microbes on both their cuticle and within their digestive tract (indicating feeding) that includes some fruit rot pathogens. This suggests that flies may be involved in vectoring fruit rots onto berries. Their association and persistence may be a potential avenue to explore for management of both pests together. Lewis’s findings related to SWD’s antagonistic and synergistic relationships with microbes may lay the foundation for improved control methods in the future, such as more effective pest surveillance methods, the development of an attract-and-kill trap using attractive volatiles, or the eventual development of a biopesticide, or a pesticide derived from natural sources. In the meantime, however, Lewis also embarked on a project that will likely have more immediate impact for farmers by evaluating and making small changes to pesticide application- currently a common management practice. Insecticide sprays are one of the few current management options available for SWD, so there is a strong impetus for optimizing use to be as ecologically and economically sustainable as possible. Caneberries like blackberries and raspberries have dense foliage and fruit are dispersed throughout, so it is important that insecticides reach all areas of the plant to be effective. Spray coverage was evaluated on commercial berry farms in Maryland, using farmers’ equipment and techniques, and dispersal of the insecticide through the plant canopy was found to be highly variable. Specifically, the interior and lower parts of the plant had poor coverage, leading to potential places for flies to avoid insecticide [4]. These are also areas of the plant with high rates of egg deposition. ![Figure 3. Adapted from Lewis and Hamby, 2020 [4]. Lewis placed photopaper cards in the exterior and interior of caneberry foliage at 3 heights, and sprayed dye at two different rates. Percent coverage was low at the lowest heights (red box), lower volume (blue boxes), and within the interior of the canopy (green box). Lack of pesticide exposure to the lower canopy and interior canopy may create refugia for adult SWD to avoid insecticides.](/uploads/4/4/1/3/44130801/lewisblog-photo3_orig.png) Figure 3. Adapted from Lewis and Hamby, 2020 [4]. Lewis placed photopaper cards in the exterior and interior of caneberry foliage at 3 heights, and sprayed dye at two different rates. Percent coverage was low at the lowest heights (red box), lower volume (blue boxes), and within the interior of the canopy (green box). Lack of pesticide exposure to the lower canopy and interior canopy may create refugia for adult SWD to avoid insecticides. With laboratory experiments and a four-year field trial, Lewis and her colleagues explored ways to optimize spray coverage on small diverse Maryland farms. First, a bioassay in the laboratory demonstrated that Mustang Max™, a commonly used pesticide, effectively reduces larval SWD infestation rates in raspberries regardless of spray coverage levels. But, higher spray coverage is important for effectively killing adult flies [4]. Experiments in the field demonstrated spray coverage rates were highly variable across different equipment types and application rates [4] (Figure 1, Figure 3). Lewis found that a small behavioral change, such as calibration of equipment specifically for caneberries, can have a considerable impact on insecticide effectiveness. On small diversified farms like those common in Maryland, farmers often use the same sprayers to treat multiple fruit and non-fruit crops with varying canopy structures and heights. By making small adjustments to the equipment, sprays can be directed at caneberry foliage, reducing overhead drift and increasing coverage on the target plant canopy. This easily implemented and low-cost change can be highly beneficial for stakeholders.
During her time at UMD, Maggie Lewis won numerous internal and external awards and funding, formed strong relationships with stakeholders, and was an active member of our community. You can find her @EntoMags. Immediately following her seminar, Maggie successfully defended her dissertation. Congratulations Maggie! Huiyu Sheng is a master student in the St. Leger Lab, studying the phenotypic and genomic differences of several Metarhizium robertsii strains regarding their plant promotion ability. Arielle Arsenault-Benoit is a PhD student in the Fritz Lab, studying the influence of environmental heterogeneity on community assemblages and landscape genomics of Culex pipiens on an urban to rural gradient. References: [1] M T Lewis, E E Koivunen, C L Swett, K A Hamby, Associations Between Drosophila suzukii (Diptera: Drosophilidae) and Fungi in Raspberries, Environmental Entomology, Volume 48, Issue 1, February 2019, Pages 68–79, https://doi.org/10.1093/ee/nvy167 [2]Lewis, M.T., Hamby, K.A. Differential Impacts of Yeasts on Feeding Behavior and Development in Larval Drosophila suzukii (Diptera:Drosophilidae). Sci Rep 9, 13370 (2019). https://doi.org/10.1038/s41598-019-48863-1 [3] Rohlfs, M. and Hoffmeister, T.S. (2005), Maternal effects increase survival probability in Drosophila subobscura larvae. Entomologia Experimentalis et Applicata, 117: 51-58. https://doi.org/10.1111/j.1570-7458.2005.00334.x [4] Lewis, M.T. and Hamby, K.A., 2020. Optimizing Caneberry Spray Coverage for Drosophila suzukii (Diptera: Drosophilidae) Management on Diversified Fruit Farms. Journal of Economic Entomology, 113(6), pp.2820-2831. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290


 RSS Feed
RSS Feed




