|
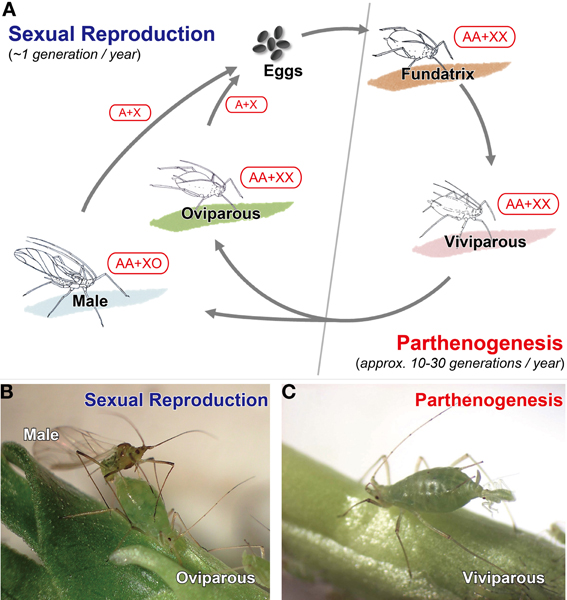
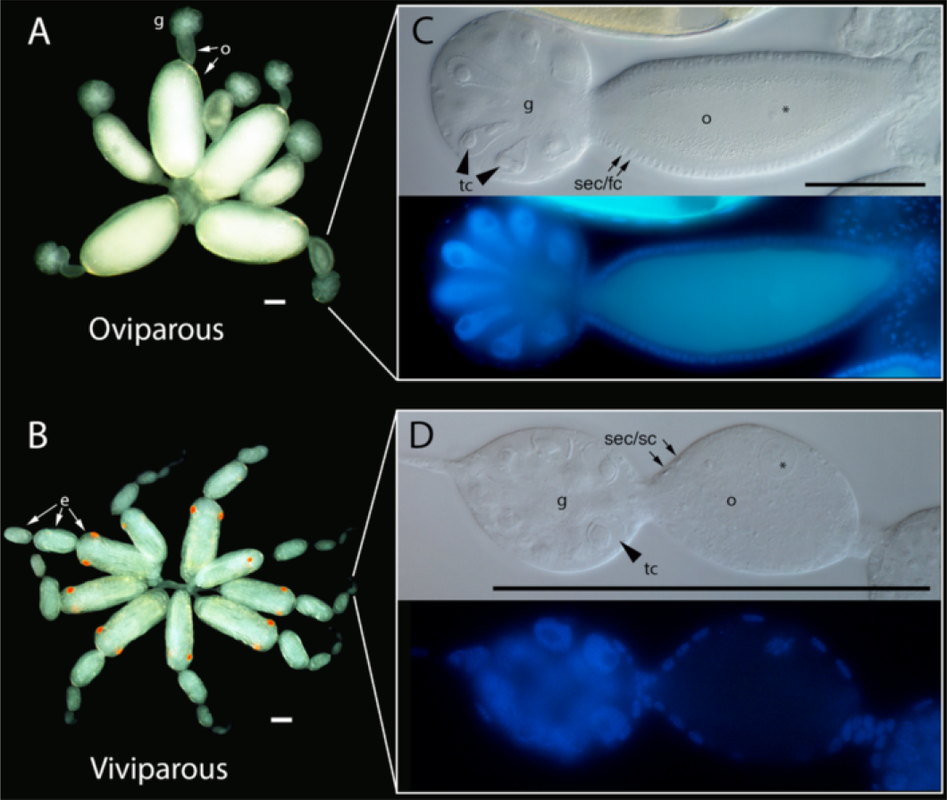
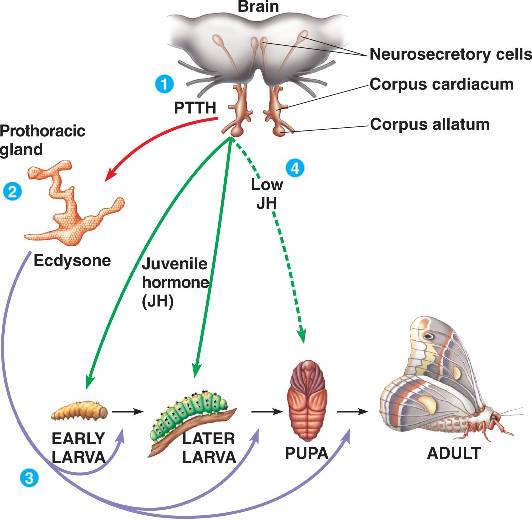
Post by Justin Rosenthal and Nathalie Steinhauer The Challenges of Optional Sex: the case of reproductive polyphenism in aphids Insects lay eggs, right? Well, in aphids, a speciose family of the Sternorrhyncha, females actually have the option of laying eggs or producing live young from embryos (instead of laying eggs). This is an example of reproductive polyphenism. Polyphenism is the ability of some organisms to adapt their phenotypes in response to environmental cues (Figure 1, from Ogawa and Miura 2014). In aphids the two reproductive strategies are a response to changes in photoperiod (day length) and this polyphenism is observed ubiquitously in these insects. In their life cycle, after having survived the winter as frost-resistant eggs, founder females reproduce asexually birthing live young that will produce further asexual females. Asexual females are all about high reproductive rates and dispersion. Those asexual females actually exhibit a second type of polyphenism in that some of them can develop wings promoting dispersion when its current location is experiencing crowded conditions. As day length gets shorter, indicating the coming of winter, sexual egg-laying females are produced (Figure 1). But how are those changes mediated by the environmental signals and how does the system switch from sexual to asexual reproduction? In last week’s colloquium, Gregory Davis illustrated the complexity of deciphering the mechanisms behind reproductive polyphenism using aphids as his model. Much of this work is available in his most recent review paper (Davis, 2012). He believes that such a novelty may have developed because an initial, slight change in morphology may have stimulated a modification of life history, with changes in life history feeding back to further changes in morphology, continuously playing off each other until obvious changes in body structure and development evolve. This production of asexual females is made possible by several important changes in how the female gametes are formed in the ovaries. In aphids we see alterations in cell divisions during meiosis, including the omission of a second round of reducing divisions (which usually divide the amount of unique DNA in half), as well as the absence of recombination between chromosomes (which normally occurs to increase variation within a population) (Blackman 1978; Hales et al 2002). Because of those changes, the gamete produced by the female already contains all the genetic material needed for its development and does not need to be fertilized to develop into an embryo. This is called parthenogenesis. An additional facet of parthenogenesis in aphids is the alternative pathway by which active centrosomes, important subcellular structures that help move DNA during cell division, are produced in asexual females. Although they are usually supplied by the sperm cell, aphids have solved this by simply generating these within the egg itself (Riparbelli et al 2005). A third obstacle that aphids had to overcome was the removal of the meiotic arrest and completion of cell division. Usually when gametes are being formed, the process stops midway until they receive signals to continue and form the final stage of the egg. In most insects, these signals are either sperm passage into the oocyte or physical oviposition. However, parthenogenetic aphids undergo neither of these and therefore are postulated to bypass arrest of meiotic progression. Later in the season, as a reaction to the reduction in the photoperiod, the asexual females will start producing a new type of aphid: sexual females, who each produce haploid gametes with a single X chromosome, mate with males, and produce fertilized eggs that enter diapause and overwinter. This change in phenotype is associated with deep morphological changes: while sexuals are oviparous (they lay eggs), asexuals are viviparous (the embryos develop inside its mother) (see Figure 2, from Bickel et al., 2013). In asexual females, embryos develop within the ovaries, and even start reproducing themselves before being born! Figure 1: Alternation of sexual and asexual phases in aphids.Figure 2: Ovaries of sexual and asexual female pea aphids. Greg Davis, along with his students, has been working to uncover how an environmental cue perceived by the mother can be signaled to the embryo to specify its reproductive fate. By experimentally exposing an asexual female to a changing photoperiod (short day then long day), it can be demonstrated that the embryos in the female were responsive to the maternal signal only during a specific window of time. The youngest embryos responded to the change in signal and developed into asexuals, while the older embryos continued on their developmental trajectory as sexuals. But what molecular pathway actually triggers the change in developmental fate? It has been previously observed that addition of juvenile hormone (JH) is sufficient to induce an asexual fate, even under the long photoperiod usually associated with sexual fate. JH is a hormone that regulates, among other things, the development of insects. It is particularly important for ensuring the full growth of larvae by preventing metamorphosis in the first couple of moltings (See Figure 3 for the classically described function of JH) (Corbit and Hardie 1985). To test the effect of the removal of JH, they used a plant derived compound, precocene, which acts by destroying cells of the corpora allatum, the endrocrine gland responsible for producing JH. However, interfering with JH has other consequences, such as the retention of juvenile characters and the interruption (called stalling) of the molting cycles at the 3rd instar. Indeed, asexual mothers exposed to long day photoperiods (which should therefore produce more asexual offspring) switched to producing sexuals when exposed to precocene (and also exhibited stalling). Interestingly, when applying precocene to sexual mothers exposed to short day photoperiods, no stalling effect could be detected in her offspring. Together these observations may suggest that it is embryonic levels of JH that are responsible for the specification of reproductive fate, rather than maternal levels of JH. Figure 3: JH and molting phases in insectsBut what is the evolutionary history of this new asexual phase? Another aspect of Dr. Davis’ work involved measuring gene expression patterns between the viviparous and oviparous eggs in an attempt to contrast these two different modes of oocyte (egg cell) development. Indeed, they found some significant differences. The gene torso-like (tsl), typically needed for proper patterning in the insect-model Drosophila, was expressed in the somatic cells but not to asexual eggs. Another gene, rolled (rl), which encodes a protein kinase that has an important role in Drosophila zygotes (fertilized eggs), was also expressed differently between the two types of eggs. These two observations point to differences in the molecular mechanisms governing the development of oviparous vs. viviparous egg development, differences that presumably evolved after the evolutionary acquisition of viviparity in the aphid lineage. Aphids are characterized for their “superfecundity”, or high reproductive capacity, resulting from the viviparous mechanism of asexual reproduction. Aphid response to the environmental cues, probably signaled through embryonic JH, allows the production of many individuals ready to spread during the optimum season while maintaining the advantages of sexual reproduction when weather becomes less ideal. Though the addition of an asexual phase for a sexual organism seems incredibly complex, Dr. Davis illustrated for us how such evolutionary transitions can be better understood by decoding the mechanisms behind such phenomena, increasing our understanding of how organisms can become so successful. References: Bickel, RD; Cleveland, HC; Barkas, J; Jeschke, CC; Raz, AA; Stern, DL; Davis, GK. 2013. The pea aphid uses a version of the terminal system during oviparous, but not viviparous, development. EvoDevo, 4(1): 10. DOI:10.1186/2041-9139-4-10 (http://www.evodevojournal.com/content/4/1/10) Corbit TS and Hardie J. 1985. Juvenile hormone effects on polymorphism in the pea aphid, Acyrthosiphon pisum. Entomologia Experimentalis et Applicata 38: 131-135. Davis, GK. 2012. Cyclical Parthenogenesis and Viviparity in Aphids as Evolutionary Novelties. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 318(6): 448–459. DOI:10.1002/jez.b.22441 (http://onlinelibrary.wiley.com/doi/10.1002/jez.b.22441/abstract) Ogawa K and Miura T. 2014. Aphid polyphenisms: trans-generational developmental regulation through viviparity. Front. Physiol. 5:1. doi: 10.3389/fphys.2014.00001 About Nathalie: Nathalie Steinhauer is a PhD student working in the vanEngelsdorp lab. She links the risk factors associated with beekeeping management to colony mortality.
About Justin: Justin Rosenthal is a PhD. student working under Dr. Jian Wang and studies the genetic mechanisms of Drosophila nervous system development, specifically the rewiring of the learning centers during metamorphosis.. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290

 RSS Feed
RSS Feed




