 Written by: Katie Reding, Serhat Solmaz, and Arielle Arsenault-Benoit Adaptation to abiotic stressors and environmental change is imperative to survival in a rapidly changing world. Dr. Reid Brennan, an ecological geneticist and postdoctoral scholar in the Pespeni Lab at University of Vermont, presented his research in aquatic systems to explore the genomic basis of populations’ responses to these stressors over short- and long- time periods. Dr. Brennan first spoke about research he had done as a graduate student in the lab of Andrew Whitehead at UC Davis studying populations of Atlantic killifish (Fundulus heteroclitus) in the Chesapeake Bay and Potomac River. From the headwaters to the mouth of the Potomac River, there exists a freshwater to brackish water gradient, and different populations of killifish can be found along the length of the river, suggesting that some might be more well-adapted for life in a more marine environment, while others, toward the freshwaters of the river source, more adapted for life in freshwater. How marine-living animals adapt to life in freshwater is essential to understanding the biodiversity of freshwater fishes, as a disproportionate number of fish species live in freshwater than marine environments, given that a small fraction of all the water on Earth is fresh. 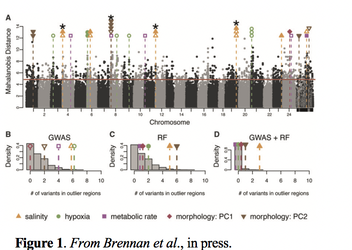 Brennan and colleagues had previously found that these brackish water-dwelling and freshwater-dwelling populations were genetically different and found physiological between them as well. Brennan conducted a genome-wide association study (GWAS) in order to associate specific genotypes with these diverging phenotypes between the two populations. An intermediate population from the middle of the Potomac River and thus the salinity gradient was also sampled. They combined GWAS with another statistical approach to scan for targets of selection. Using this approach, they found 18 loci suspected to be targets of natural selection (Figure 1) associated with phenotypic differences in the fresh and brackish water populations. Five of the loci identified in Brennan’s analyses are involved in osmoregulation, which he and colleagues had predicted to be important in the killifish’s transition from marine to freshwater. This study therefore provided strong validation that genes related to osmoregulatory processes are those most under selection during the transition from high to low salt environments. Dr. Brennan then talked about the rapid adaptation to ocean acidification in sea urchins. He pointed out another aspect of climate change in addition to the constant increase in temperatures is ocean acidification. More CO2 dissolved in the atmosphere results in higher concentrations of CO2 in oceans. This acidification causes a decrease in available minerals that some marine animals use to make their shells. Therefore, this is a selective pressure on those species. To see how these organisms will respond to this change over time, he and colleagues used a single generation experiment with the purple sea urchins. Purple sea urchins occupy the entire western coast of the North America. They also respond to experimental pH selection in the lab. Their ability to adapt makes them useful to try to understand what limits or drives the adaptation mechanism. They used two different treatments representing the two selective pressures that might occur in the wild; moderate acidification (pH 8.0), extreme events (pH 7.5). 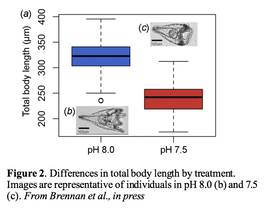 Dr. Brennan first talked about the morphological consequences of these selective pressures. Urchins under extreme conditions had lower mean body length (Figure 2) . He stated that the low pH levels stunt the growth of the sea urchins. Under different treatments the density of allele frequencies shifted towards low starting allele frequency. Meaning that although some genetic variants are rare, under different treatments, they might become adaptive. He then stated this shift towards rare genetic variation indicates that large population sizes are important for preserving this adaptive genetic variation. Current climate change models project effects beyond increased temperatures, including changes to ocean pH. Dr. Brennan and colleagues at University of Connecticut are pursuing research to explore the impact of high temperature and high carbon dioxide (CO2), concentrations on rapid adaptation and selection in copepods. In laboratory studies, copepods experienced an initial decrease in overall fitness under high temperature, high CO2 conditions. However, by the third generation, populations were rescued, suggesting adaptation over a short period of time. To explore this further, researchers used two copepod populations conditioned to either a) ambient conditions or b) high temperature and high CO2. Experimental copepod lines were then transposed, so those raised in ambient conditions were placed into high temperature and high CO2 and vise versa. A series of genetic scans on each generation yielded interesting results. The first generation of offspring were genetically similar to their parents, but by the third generation, the genetic structure of the copepod populations better reflected those initially under those conditions than to their own parental lines.  These results demonstrate that after only three generations, copepods adapted to their new environmental conditions through changes in their genetic structure. However, the mechanisms of adaptation were different for each group. In the ambient line, copepods adapted to their environment through shifts in gene expression over multiple generations. Conversely, copepods originating in the high temperature, high CO2 environment thrived in the ambient conditions due to shifts in allele frequency. This equates to an overall loss of genetic diversity in the population. So, the rapid changes undertaken to adapt to simulated climate change conditions actually reduced genetic diversity. Consequently, this population would have less ability to respond to future environmental changes. These studies demonstrate that impacts of global change on oceans could result in rapid adaptation, selection, and evolution of aquatic organisms. These changes are evident on a genomic level, even after just one or a few generations. Changes of this type and magnitude can reduce overall population genetic diversity, and potential loss of species-wide resilience to subsequent changes. Brennan, R.S., Garrett, A.D., Huber, K.E., Hargarten, H., Pespeni, M.H., (2018). Rare genetic variation is important for survival in extreme low pH conditions. BioRXiv. doi: https://doi.org/10.1101/422261 Brennan, R.S., Healy, T.M., Bryant, H.J., Van La, M., Schulte, P.M. and Whitehead, A.,. Genome-wide selection scans integrated with association mapping reveal mechanisms of physiological adaptation across a salinity gradient in killifish. Molecular Biology and Evolution. In press. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290

 RSS Feed
RSS Feed




