|
written by: Dylan Kutz, MS student, Lamp Lab Whenever we see the words “genetically modified” in the news these days, they’re usually followed by two things: the word “crops” and a lot of controversy. Genetically modified crops or “GM” crops are crops that have had their DNA altered through genetic engineering to gain a desirable trait, such as pest suppression. While some fear the effects of genetically modified crops on human health, numerous studies have thoroughly debunked the myth that GM crops are dangerous to humans. Still, much remains unknown about whether insect-resistant GMO crops affect non-target insects after harvest, or even how they degrade after crops are harvested. Veronica Yurchak, a Ph.D. student working in the Hooks Lab at the University of Maryland’s Department of Entomology got to the bottom of these after-harvest mysteries in her master’s thesis. Yurchak decided to study one of the most common and earliest adopted GM crops on the market: Bt corn. Bt corn contains genes derived from the soil bacterium Bacillus thuringiensis (hence the “Bt”). These genes produce toxins known as Cry (short for “crystalline”) proteins, which act as a highly selective poison for insect pests. Farmers plant different varieties of Bt corn to control the biggest pest they anticipate that year. In the fall after grain is harvested, stalks and leaves containing Cry proteins are left in the field. Yurchak wanted to find out how managing this crop residue affected Cry protein breakdown. Yurchak selected four common post-harvest management practices farmers use to deal with corn debris, including chisel plowing (where the soil is tilled, mixing in debris on the surface), flail mowing (where residues are chopped, but left on the soil surface), planting a cover crop, or simply leaving the harvested field undisturbed (Figure 1). Because chisel plowing incorporates corn tissue into the soil where microorganisms can degrade it, she thought it should have the fastest breakdown of Cry proteins. Yurchak planted study plots of both Bt corn and non-Bt corn which would later be harvested, and the field managed using these methods. After each post-harvest treatment, Yurchak collected corn debris from each field which she freeze-dried and ground into a fine powder. The freeze-dried corn was fed to European corn borers, a caterpillar that is a major corn pest and easy to raise for laboratory experiments. After being fed on this corn for seven days they were weighed. When caterpillars were smaller it indicated that there was more Cry protein in the corn sample because the protein reduces growth. Yurchak repeated the entire experiment in another post-harvest season two summers later. 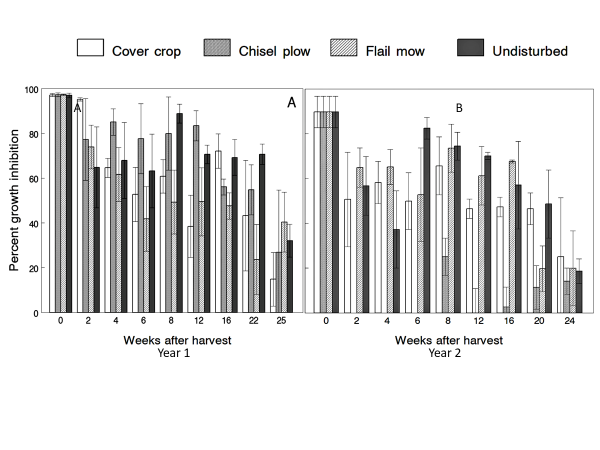 Figure 2: A bar chart showing the percent corn earworm growth inhibition as a function of time after harvest from both study years. Note the differing trends of the chisel plow treatment (2nd bar in each grouping) between both sampling years. For all treatments, the Cry protein broke down over time. Figure 2: A bar chart showing the percent corn earworm growth inhibition as a function of time after harvest from both study years. Note the differing trends of the chisel plow treatment (2nd bar in each grouping) between both sampling years. For all treatments, the Cry protein broke down over time. Upon examining her results, Yurchak noticed that corn borers fed corn debris managed using the chisel plow method were the smallest in the first year, the opposite of her hypothesis, but were the largest in the second year of the study as she had originally hypothesized (Figure 2). Conflicting results within the same treatment such as this are often the most exciting findings in an experiment, as they suggest other forces are at work in the experiment. The difference in degradation between the two sampling years can be attributed to several different factors of the study. Chisel plowing a field mixes the corn debris into the soil, which can potentially cause it to break down faster. However, the sampling protocol between the two study years differed, and it is likely that the inhibition in degradation seen during the first year was due to the design of the litter bags used to facilitate tissue sampling throughout the winter. The litter bags the first year were packed very tightly and made of a fine mesh, likely restricting contact between the tissue and surrounding soil environment. In the first year Yurchak collected corn tissue from tightly-packed sacks buried in the soil which may have prevented soil-debris interaction. Changes over time in the microbial community of the corn field soil could also possibly change the results from year to year. Additionally, temperature differences between the two winters could also have changed the rate at which toxins were being broken down, as temperatures below four degrees Celsius are known to stall microbial degradation. The differences in the two years show that there are more paths to explore in understanding Cry protein breakdown. Overall, Yurchak’s study on post-harvest Bt corn degradation has informed ecologists and farmers on what practices and conditions degrade Bt toxins the fastest, which could play an important role in keeping track of Bt toxins in cropland. Ultimately, this research could help farmers avoid unintended impacts on non-target organisms. About the Author Dylan Kutz is a master’s student working in the Lamp lab at the University of Maryland’s Department of Entomology. His research focuses on agricultural drainage ditches as sources of spider diversity for neighboring croplands. Additional Research by Veronica Yurchak Rosario-Lebron, A., Leslie, A. W., Yurchak, V. L., Chen, G., & Hooks, C. R. (2019). Can winter cover crop termination practices impact weed suppression, soil moisture, and yield in no-till soybean [Glycine max (L.) Merr.]?. Crop protection, 116, 132-141. Comments are closed.
|
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290


 RSS Feed
RSS Feed




