|
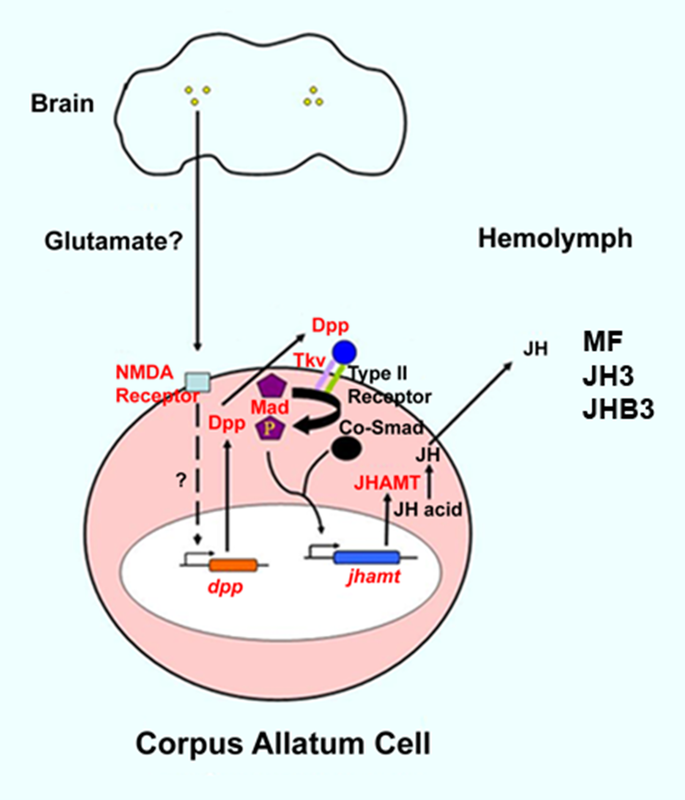
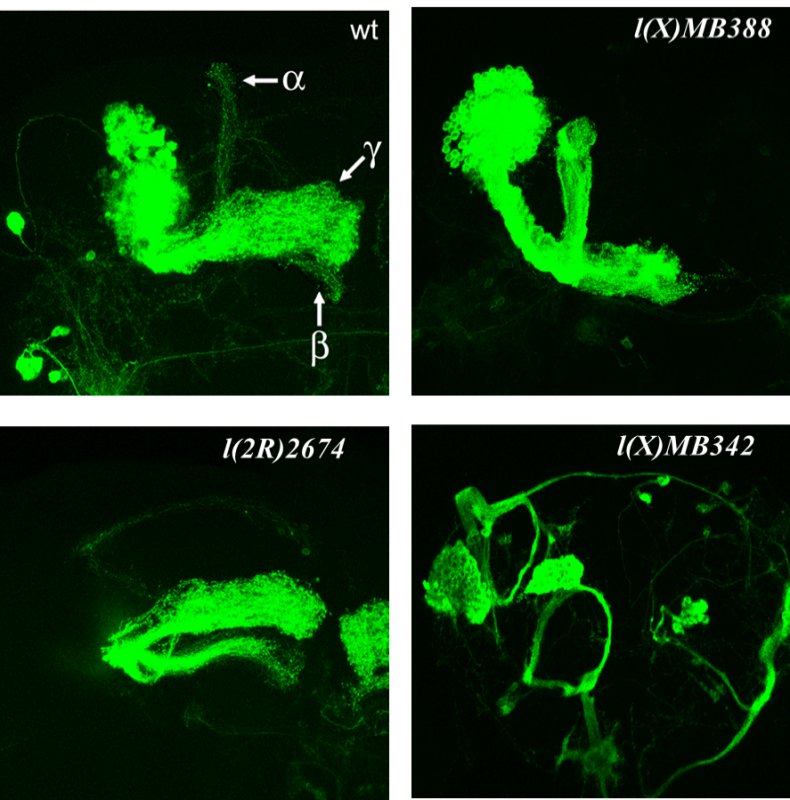
The generation of a mature insect from its larval stage might seem unimaginable at first. For instance, how can a worm-like larva transform into a winged butterfly? They are completely different, not only in their appearance but their physiology and behavior as well. With the correct cellular and molecular mechanisms, however, the entire process of metamorphosis can be properly coordinated, often in a swift and timely manner. This, and questions concerning the details of this phenomenon, is precisely the subject of study for Dr. Jian Wang. After receiving degrees at Nanjing Agricultural University andShanghai Institute of Entomology, Dr. Wang went ahead to investigate the aforementioned topic, with a concentration specifically in the laboratory fruit fly, known as Drosophila melanogaster. The “lab rat” Drosophila, while a useful genetic model, can also serve as a model for insect physiology. Thus, this animal can provide crucial information on the function of genes and signaling pathways that are often, and conveniently, transferrable to their vertebrate counterparts. At the same time, scientists who study Drosophila can apply novel information learned about insect hormonal changes, reproduction, behavior etc. to insects at large, thereby providing a centralized method for studying these invertebrates as a whole. 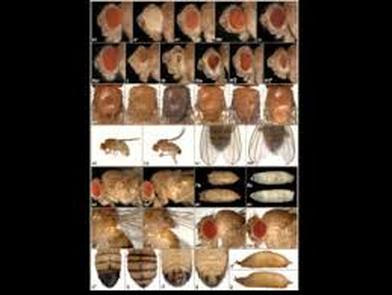 The image above is a compilation of just a few different genotypes/phenotypes of Drosophila; the specialized field of uncovering what different genes do, many times by creating mutations and observing the resulting phenotypes, is known as functional genetics/genomics. (http://www.drosophila-images.org/2006.shtml) One of the main questions that Dr. Wang is interested in solving is the pathway of Juvenile Hormone (JH) signaling, including the identification of signaling molecules and receptors that compose this pathway. Initial evidence showed that the ablation, or gross removal, of a neuroendocrine structure known as the corpus allata (CA) resulted in pupae that would die prematurely before they could emerge as adults. Thus, the CA was identified as a major determinant in insect metamorphosis. Next, a series of experiments showed that when the CA was destroyed, it was possible to partially rescue the lethal developmental effects by adding any of 4 different “Juvenile Hormones”, 3 naturally-occurring hormones and the other a well-known artificial analog of this hormone. Eventually, a complete picture was formulated for the juvenile hormone pathway: the brain stimulates the cells of the CA with neurotransmitters to produce dpp, a morphogen involved in development; and this molecule activates the TGF-β signaling pathway to produce JHAMT, an enzyme involved in the synthesis of any of three juvenile hormone isoforms. The other main topic of Dr. Wang’s research is the function of genes that are important to the development and maintenance of the nervous system. Much of this work uses a genetic mosaic technique, which can generate parts of an animal that are completely mutant for a particular gene. One well-studied brain structure in the Wang lab is the mushroom body. Critical to formation and retrieval of olfactory memory, the mushroom body is a structure composed of bundles of dendrites and axons. Ubiquitous in insects, and possessed by some non-insects as well, this suborgan has a characteristic V-shaped axonal branching pattern. The degeneration and/or malformation of these axonal lobes has been under intense study in the Wang lab, whereby the function of a gene can be deduced by the aberrant resultant phenotype observed following the generation of a mutation for that mushroom-body expressing gene. Of particular interest in the past decade or so was the function of DSCAM, a protein involved in neuron synaptic formation, the precise location where two neurons meet and communicate at the molecular level. A multitude of mutations were generated for the gene producing this protein which resulted in two important discoveries. Firstly, a DNA sequence analysis showed that the Drosophila gene producing DSCAM is conserved, in terms of nucleotide base pairs, with a gene found in humans. Secondly, through a clever transgenic assay, it was found that pupation rate, and to a lesser extent eclosion rate, could be partially rescued by overexpressing different forms of human DSCAM in mutant flies unable to produce any DSCAM of their own. These two experiments provided evidence that human and Drosophila DSCAMs not only have a conserved sequence, and are possibly evolutionarily related, but that they are functionally conserved as well. Currently, the Wang Lab is continuing its quest to uncover more genes that play a critical role in the development of the Drosophila nervous system, using the mushroom body as the experimental tissue of choice. The senior graduate student, Lijuan Du, is currently investigating the overlap between the Hippo pathway and the JAK/STAT pathway. Over the years, she has found evidence that one particular gene is regulated downstream by both of these pathways. These pathways are crucial in regulating the cell cycle, carefully guiding cells through proper rates of cell proliferation and programmed cell death. The other member and writer of this blog, Justin Rosenthal, is beginning his second year in the Wang Lab. The focus of his research is the function of a single gene, Darkener of apricot(Doa), in ensuring neuronal viability through the drastic changes of metamorphosis. He has already confirmed work done by previous students that this gene is necessary for survival of the y-lobe of the mushroom body and is now devising experiments to test the role of the individual variants of this gene and the functional importance of some of the exons, or the coding parts of the gene. 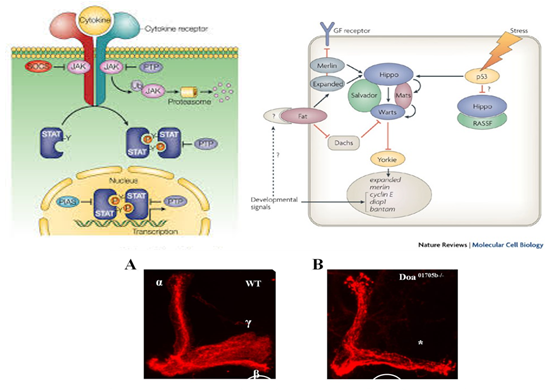 The two top images show the JAK/STAT (left) and hippo(left) pathways, delineating the intricacies of each. The bottom photo is of two mushroom bodies, visualized with a red fluorescent marker. The left is the wt condition and the right it the mutant condition. One can easily see the stark differences, wherein the mutant mushroom body is completely missing the medial-branching y-lobe(top left: http://www.nature.com/nri/journal/v3/n11/full/nri1226.html), (top right: http://www.nature.com/nrm/journal/v8/n8/fig_tab/nrm2221_F3.html) (bottom: Qiong Yao) Article published by Dr. Wang and others: http://www.sciencedirect.com/science/article/pii/S096517481100110X Article related to neuron patterns in Drosophila:http://www.nature.com/nature/journal/v497/n7447/full/nature12063.html About Justin:
Justin Rosenthal received his undergraduate degree from the University of Maryland-College Park in 2011 in the Biological Sciences, with a concentration in neurobiology/physiology. Upon beginning his PhD. Program here, Justin began investigating the role of a particular gene, darkener of apricot(Doa), in promoting neuron survival through the pupal stage of insect life, i.e. metamorphosis. Building upon previous research, it became ever more convincing that without this gene certain neurons within aDrosophila’s brain will not survive until adulthood. Currently he is working out the purpose of specific exons and isoforms of this gene, as several variations exist. Further research will likely include expansion of this investigation into other non-nervous tissue. Overall this information will provide a molecular model for how cell death, especially in neurons, proceeds. Post by Samuel Ramsey and Rebecca Wilson In previous colloquia this semester, we’ve had the pleasure of learning about research focusing on a variety of different arthropods, ranging from potato leafhoppers to harvestmen. However, our most recent colloquium deviated slightly with a presentation focusing on entomopathogenic fungi and their potential to control the spread of deadly vector-borne illnesses. Dr. Raymond St. Leger started his presentation in the most well attended colloquium of the semester thus far with a video showcasing a diverse array of stunningly bizarre Cordyceps fungi. Many are known to have a profound and often grotesque effect on their infected insect hosts, with some well known for influencing insect behavior. Entomopathogenic fungi boast great promise as biological control agents due in no small part to their strong host-specificity and staggering diversity. Dr. St. Leger explained that are species of fungi that specialize in killing basically every species of insect. This list includes the mosquitoes responsible for vectoring malaria, a disease that causes more than 1 million human deaths annually. As the mosquito and its Plasmodium parasite is the cause of inimitable human suffering, help from this fungus is entirely welcome. Unfortunately, Cordyceps prefer to grow inside of their host insect, making them rather difficult to culture in labs. The St. Leger lab has circumvented this issue by working with two anamorphs of Cordyceps, Beauveria and Metarhizium. They have lost their sexual phase, and in so doing, are a lot less temperamental. Metarhizium, which occurs frequently in nature as a plant symbiont, initially showed promise as a biological control agent against mosquitoes, but lacked the necessary virulence to kill them quickly enough to prevent the many bites that spread malaria. As St. Leger explained to us, it is not naturally in a pathogen’s interest to exterminate its host species. Mosquitoes infected with Metarhizium, in many cases, were able to feed and even mate before succumbing to their fungal parasite. By super-charging these fungi with proteases, the St. Leger lab was able to significantly decrease the amount of time required to kill mosquitoes and suppressed their appetites, in the interim, even in the presence of potential hosts. Another undertaking in the lab involves producing a fungus that is able to kill the causative agent of malaria inside of the mosquito’s hemolymph and preventing its spread into the salivary glands, thereby curing the mosquito to render her harmless upon her next blood meal. Clearly, the St. Leger Lab has been busy, as they were able to transgenically express a scorpion toxin and that of a funnel web spider in the fungus to greatly increase its virulence. Dr. St. Leger and his graduate students are also involved with developing fungi that can remedy other insect related human health and welfare issues, including famine. They’ve worked with developing and mass-producing strains of Metarhizium that may reduce populations of locusts and coffee borer beetles. Research on this astounding group of fungi and their use in biological control is currently being advanced worldwide. It was great to learn that our own Dr. Raymond St. Leger continues to work at the forefront of this vital field. Fang W, Vega-Rodriguez, J., Ghosh, A.K., Jacobs-Lorena, M., Khang, A and St. Leger, R.J., 2011. Development of transgenic fungi that kill human malaria parasites in mosquitoes Science 331: 1074-1077. Samuel Ramsey is a 2nd year PhD student in the Shrewsbury lab currently studying competition in egg parasitoids of the brown marmorated stink bug.
Rebecca Wilson is a 1st year master’s student in the lab of Dr. Bill Lamp. She is broadly interested in aquatic entomology and is currently studying larval black fly distribution in western Maryland. Last week, our department welcomed visiting scientist, Dr. Nora Underwood to present her lab’s research at our weekly colloquium seminar. And although Dr. Underwood hails from Florida State University, whose football team defeated the Terps 63-0 in a record setting game a few short weeks ago, she was warmly received by a packed house here at the University of Maryland. This is surely due, in no small part, to Dr. Underwood’s connection to two of our own who came to us from her lab. Graduate student, Alex Forde (Gruner Lab) and post-doc Amanda Buchanan (Hooks Lab) both hail from the Underwood lab as former lab tech and graduate student, respectively. However, the real draw for most of the crowd was surely the topic of plant-insect interactions, which is an area of research shared by many labs within our own department. Dr. Underwood’s presentation came to us in two parts, and although there was an admission of disconnect between the topics, there is one theme that bridges the divide, and that is an addiction to pursuing questions with huge, field-based studies. The first half was a discussion of a large study exploring mechanisms of changes to a plant population resulting from damage inflicted by insect herbivores. Every farmer, gardener, and even grad student knows that plants don’t grow too well when insects are eating them. However, this study seemed to stem from being dissatisfied with simply acknowledging that insect herbivores are bad for plant populations, and the need to know exact reasons why insect herbivory might cause plant populations to decline. To address these questions, Dr. Underwood and colleagues experimented with populations of horsenettle (Solanum carolinense), measuring growth, fecundity, asexual reproduction, and rates of herbivory over two years for each individual plant, all while manipulating plant densities and herbivore abundances. The idea behind measuring all of these demographic factors rather than simply measuring a change in biomass was to get at how and not just whether insect damage leads to change in the population. For example, a perennial plant population may decline from one year to the next because some plants were killed, because there were fewer energy reserves in the roots of overwintering plants, because there was decreased seed production, or because there were fewer “runners” produced asexually. To be able to answer these questions, though, requires compiling a massive dataset of all these demographic factors, and then using stage-structured demographic models to show how each factor is affected by different levels of herbivory. For more information on this study, see Dr. Underwood’s publication in the journal Ecology (Underwood & Halpern 2012). 
Dr. Nora Underwood’s research involves following the growth and development of each individual in populations of this plant, Carolina horsenettle (Solanum carolinense), in order to learn about how effects of plant density and insect herbivory interact. Photo Credit: Ted Bodner, Southern Weed Science Society, Bugwood.org
The second half of the talk introduced a new framework for studying associational effects within plant communities. Associational effects account for how the struggle for plants to avoid being eaten by insect herbivores occurs within a complex matrix of other species of plants, and that plant insect interactions involve more than just the interaction of the insect and the focal plant. In other words, if you are a plant, how susceptible you are to herbivores may depend on who your neighbors are? The effects that neighboring plants can have on the species in focus have been described many different ways. For example, neighboring plants may produce volatile chemicals that limit an insect’s ability to find the focal plant, which would result in lower herbivory rates. On the other hand, neighboring plants may serve as a host to many species of herbivores that also feed on the focus plant species, making it more susceptible to attack from herbivores. Dr. Underwood presented a novel framework for studying these kinds of effects on herbivory rates between neighboring plant species as a way to tease apart mechanisms underlying the effects. In doing so, Dr. Underwood has also signed up her lab for even more large, field based experiments, because to differentiate between effects of density of the focal species and density of the neighboring species, both have to be changed simultaneously. Fortunately, horsenettle is probably more than willing to oblige, as it is a noxious weed throughout much of the United States. This fact also helps the Underwood lab to walk the thin line between basic and applied ecological research, as the results of these experiments are useful not only from the standpoint of our basic understanding of how plants and insects interact in nature, but in informing us of ways to manipulate the landscape to reduce damage to focal species like crops, or ornamentals. More information in this new framework can be found in a new publication by Dr. Underwood and others, which will be out some time next year (Underwood et al. 2014). Underwood, N. and S. Halpern. 2012. Insect herbivores, density dependence, and the performance of the perennial herbSolanum carolinense. Ecology 93(5): 1026-1035. Underwood, N., B.D. Inouye and P.A. Hambäck. 2014. A conceptual framework for associational effects: when do neighbors matter and how would we know? Quarterly Review of Biology: in press. About Alan:
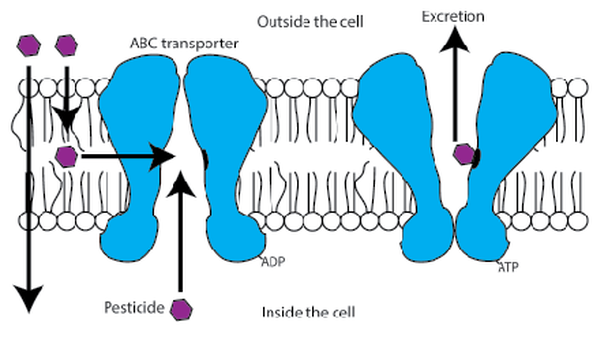
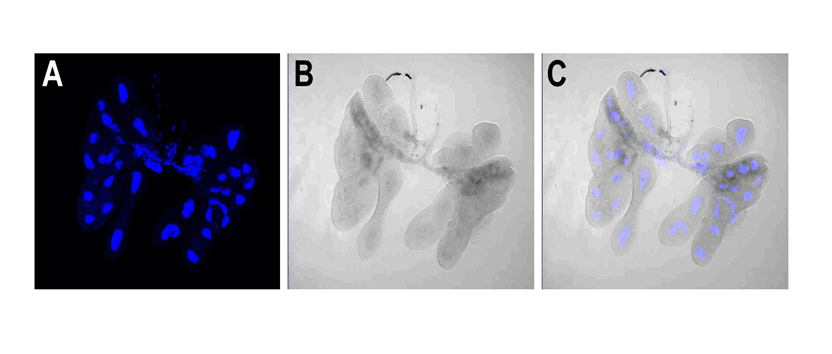
Alan Leslie is a Ph.D. candidate in the Lamp Lab, studying aquatic macroinvertebrates and their effects in regulating ecosystem functions. His research project is focused on determining the effect that burrowing aquatic invertebrates have on nutrient transport in agricultural drainage networks. Post by Ryan Gott and Nathalie Steinhauer Colloquium this week was a special treat as Grace Kunkel defended her Master’s thesis “Investigatingin vivo honey bee toxicology and whole honey bee hive dynamics using fluorescent dyes.” Grace’s work focused on two different levels related to honey bee health: the individual honey bee and the entire honey bee colony. Collapsing colonies have been in the headlines since 2006, and with finger-pointing at many possible perpetrators. These potential causes of Colony Collapse Disorder (CCD) include bee parasites, bacterial and viral diseases, fungi, poor nutrition, and chemicals (but not cell phones!). While current research suggests that CCD is most likely caused by a combination of these factors, Grace decided to focus on how chemicals may be affecting the honey bees. While the most suspect chemicals include pesticides and fungicides, using these chemicals in experiments is complicated and expensive. As a solution Grace used dyes that were chemically similar to specific pesticides. This allowed her to not only save money, but also easily track the dyes though individual bees and colonies. When honey bees are exposed to pesticides, proteins called ATP-binding cassette transporters (ABCs) form the first line of defense by actively sequestering the chemicals in the honey bee’s gut to be excreted (Fig. 1). If these ABCs are inhibited, pesticides can build up to dangerously toxic levels in the honey bee’s blood. Grace found that both excretion by ABCs and inhibition of ABCs can be studied in individual honey bees using dyes that mimic the behavior of pesticides. If honey bees encounter ABC inhibitors in their environment, this could sensitize them to pesticides. The dynamics of a chemical in a whole colony, however, can be vastly different from those in an individual bee. When chemical exposure in a honey bee colony is explored, typically the chemical is applied directly into the hive through either sugar syrup or pollen patties. Grace questioned whether those two methods of chemical delivery were truly equivalent or if they lead to different distributions of the chemical in the colony. To explore this she once again used dyes as chemical surrogates. By incorporating dyes into syrup and patties, she could track their movements through each component of the colony, from honey to wax and from larvae to adult bees (Fig. 2). Dye delivered in sugar syrup gets stored and accumulates in the hive, while dye in pollen patties does not. Grace believes this shows that pollen patties are directly eaten by the bees and not stored, unless the patty is eaten while the bees are producing royal jelly. Royal jelly is a highly nutritious substance produced to feed future queens, so chemicals that end up in royal jelly could potentially affect future generations of bees. Grace’s findings have important implications for colony health. Behavior of expensive pesticides in a honey bee colony can be mimicked using similar dyes for a fraction of the cost. Delivery of chemicals through pollen patties equates to an “acute” exposure to a chemical, while the stored syrup would be a “chronic” exposure. This means the method of chemical delivery makes a big difference, and future researchers using actual pesticides in colonies should choose their delivery method wisely. About Nathalie: Nathalie Steinhauer is a PhD student working in the vanEngelsdorp lab. She studies the risk factors associated with beekeeping management linked to increased honey bee colonies mortality.
About Ryan:Ryan Gott is a PhD student in the lab of Bill Lamp. Ryan studies environmental toxicology and environmental risk assessment with a focus on developing biomarkers for chemicals that interact with ABC transporters. Post by Chris Taylor This week’s Colloquium was the first of the semester where a grad student in our department defended their dissertation. Bridget DeLay’s work, “Symbionts associated with the salivary glands of the potato leafhopper, Empoasca fabae, and their function when feeding on leguminous hosts” was a particularly interesting topic for me since I work with the symbionts of the brown marmorated stink bug. Mutualistic associations between animals and microbes have been documented in many taxa, so it is not surprising that these relationships are found in a diverse assemblage of insects. The field of insect-microbe interactions is a fast growing and exciting branch of entomology. With the relatively recent increase in the development and availability of genetic tools for identification and sequencing, more and more of these relationships are being uncovered. We have barely scratched the surface in terms of understanding the scope of these relationships and the roles that they play in the biology of the host organism. The microbial symbionts of insects usually have very intimate relationships with their hosts. Therefore, these symbionts are usually obligate (association is required for survival), vertically transmitted (parent to offspring), and have a reduced genome. They have a variety of functions, one of the most prominently studied being their role in diet supplementing. Most blood, xylem and phloem feeding insects have symbionts, which aid the host by providing the essential amino acids or vitamins that their nutrient-deficient diets cannot provide. Most described symbionts have been found in the guts of their hosts, but recently they have also been isolated from the salivary glands of some insects. The potato leafhopper is a highly polyphagous, native agricultural pest that causes a condition known as ‘hopper burn’ (here and here), which causes yellowing and stunting of the plants on which it feeds. Although Paul Buchner, one of the most well known researchers of insect-microbe interactions, referred to the Auchenorrhyncha (the suborder of insects to which the leafhoppers belong) as a “fairyland of insect symbiosis,” Bridget was the first to look at a cicadellid leafhopper to determine its symbionts. She identified two: a species of Wolbachia and a species of Sulcia. By using antibiotics to produce aposymbiotic (symbiont-free) leafhoppers, she investigated survivorship, fecundity, and nymphal survival when the symbionts were not present. She determined that both nymphal survival and survivorship were decreased when the symbionts were removed, indicating that the symbionts are necessary for success of the host leafhopper. Bridget also wanted to look at what role, if any, the symbionts played in feeding on plants by the leaf hopper. She allowed symbiotic and aposymbiotic leaf hoppers to feed on alfalfa and fava beans, and showed that symbiotic leaf hoppers reduced photosynthesis rates in alfalfa and fava beans as well as transpiration rates in alfalfa. In another study, she applied plant hopper saliva to the stems of plants and data suggested that the symbionts may actually suppress wound response pathways in the plants while the leaf hoppers feed. Bridget sequenced the 1st transcriptome data for cicadellid salivary glands that is now available in GenBank (see picture citation below). This work provides an insightful look into the intimacy of these insect-microbe interactions. Her research suggests the microbes aid the leafhopper in its feeding and contribute to the damage the plant endures: a very novel and understudied aspect of insect-microbe biology. With the advent of genetic identification tools, our knowledge of insect-symbiont interactions will continue to increase. This also opens up the possibility to exploit these symbiotic relationships for the management of pests like the potato leafhopper. More and more work, Bridget’s included, is showing that these associations are vital to the success of the host insect, and the disruption of this relationship may turn out to be a useful management tool for pest insects About Chris:
Chris Taylor is a PhD student with focal areas in IPM and insect-microbe symbioses. He studies the brown marmorated stink bug (BMSB), and the focus of his research is on understanding the relationship between BMSB and its gut symbionts to determine whether exploiting this relationship is feasible in management programs. Post by Brian Lovett In a world that wants to bring an end to the malaria-causing parasite Plasmodium, Dr. David O’Brochta’s lab is busily working to create more of it. By carefully altering the genome ofAnopheles stephensi, a mosquito that carries Plasmodium, he is turning these sinister insects intoPlasmodium factories. Though most people find the idea of a mosquito’s salivary gland packed full with Plasmodium sporozoites terrifying, it forms the foundation of a promising malaria vaccine to the researchers at Sanaria Inc. In his research overview seminar at the UMD Entomology department colloquium, Dr. O’Brochta details his history of using unusual means to achieve useful ends. At the heart of his research is an aim that is as old as the field of genetics: develop a “seamless understanding” of the relationship phenotype has with genotype. With the dawn of genomics and transcriptomics, the scientific world has accumulated much more information on genotype than on phenotype. Without a proper connection between these strings of genetic letters and the characteristics we see from them, we are living in a world where we know more than we understand. Drawing the connection back from genotype to the phenotype is called reverse genetics, and these connections are driven by genetic technologies. Dr. O’Brochta has developed transposon-based genetic technologies to try to connect the dots between the vast genetic information researchers now have on mosquitoes and how these genes work together to create a mosquito. Dr. O’Brochta was careful to point out that he did not re-invent the wheel: Barbara McClintockwas the first to characterize the action of transposons in her Nobel prize winning experiments on corn, and generations of Drosophila researchers have been pioneering and testing transposon-based genetic engineering for decades. Rather, Dr. O’Brochta is “making it work in new systems.” His new system is the mosquito, and his innovations using these transposons have some amazing applications. Transposons are small genetic sequences that have the ability to jump around genomes carrying with them whatever sequences they contain. Dr. O’Brochta describes transposons simply as chassis with an engine. The engine is the enzyme that allows the transposon to move about, and the chassis is the sequence that is being moved. Like cargo, Dr. O’Brochta has loaded reporter sequences onto the piggyBac transposon. The reporter sequences respond to their location along the genome and report back, often with an observable fluorescence. This system isn’t particularly useful when the transposon is constantly using its engine to jump around in the genome, so after the reporter transposon finds its home in the mosquito’s genome, the mosquitoes are crossed with wild-type mosquitoes to remove the transposon’s engine. The mosquitoes that result from this cross end up with a reporter sending out information about their precise location in the genome. With manipulation of the strength of the promoter and the splice site affinity, Dr. O’Brochta can use these reporters to discover enhancer sequences hidden along the genome and find spaces between the exons that make up genes. These studies reveal, with incredible detail, where, when and what is transcribed along the genome. The pictures below show a mosquito enhancer that was found to only drive expression of the reporter in the abdomen of female mosquitos. The identification of this enhancer allows scientists to study and express genes in the same location and at the exact same moment in mosquito development. This allows unprecedented control over transgene expression in mosquitoes. 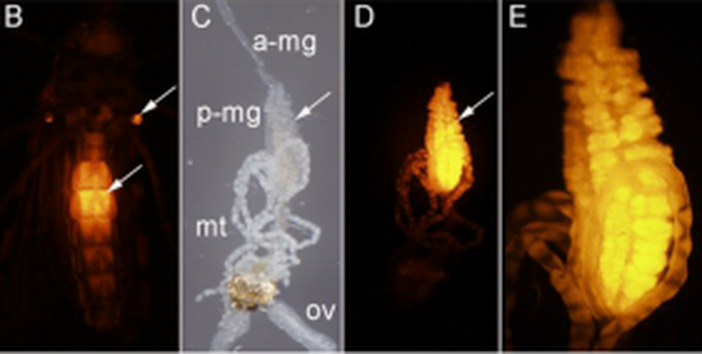
B – ventral view of an adult female under UV light showing enhancer-driven reporter gene expression in the abdomen (arrow) and halteres (arrow) (red fluorescence). C – dissected alimentary canal and ovaries (ov) of adult female shown in B; anterior midgut (a-mg), posterior midgut (p-mg), Malpighian tubules (mt) D - same as C but viewed under UV light to reveal reporter gene expression in the posterior midgut. E- higher magnification of the posterior midgut shown in D.
Dr. O’Brochta wants to use these techniques to “pop the hood and see what’s inside” of mosquito physiology and genetics. Already he has begun investigating the genes of mosquito hemocytes and salivary glands. Much can be learned about hemocyte gene expression, and many salivary gland genes have an unknown function. Due to the flexibility and high activity of the piggyBac transposon system he has developed, Dr. O’Brochta’s potential projects are only limited by the imagination. If you would like to learn more about his work, please visit his lab website. Brian Lovett is a PhD student studying mycology and genetics in agricultural and vector biology systems. He is currently working on projects analyzing mycorrhizal interactions of winter wheat and the transcriptomics of malaria vector mosquitoes
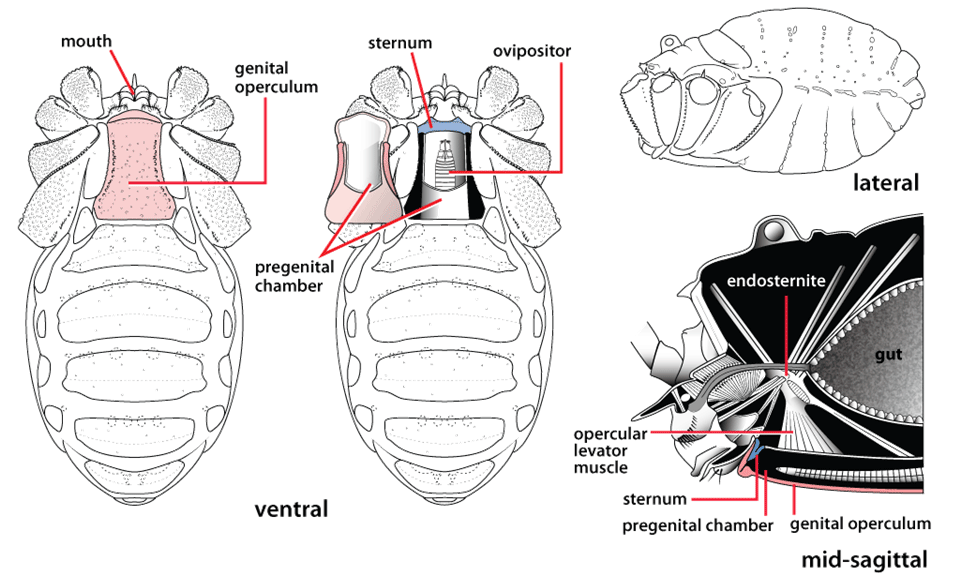
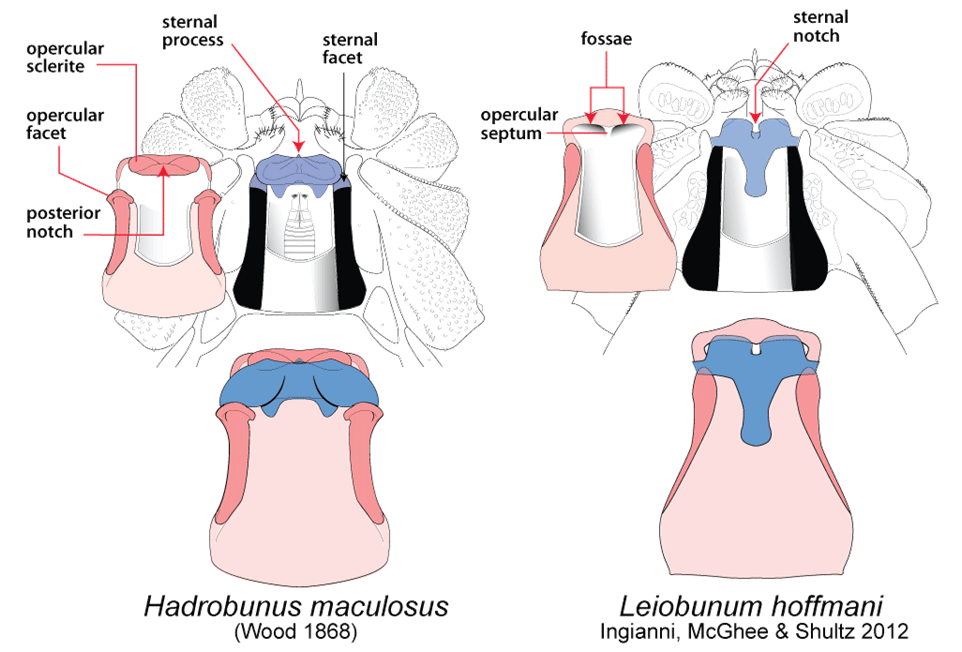
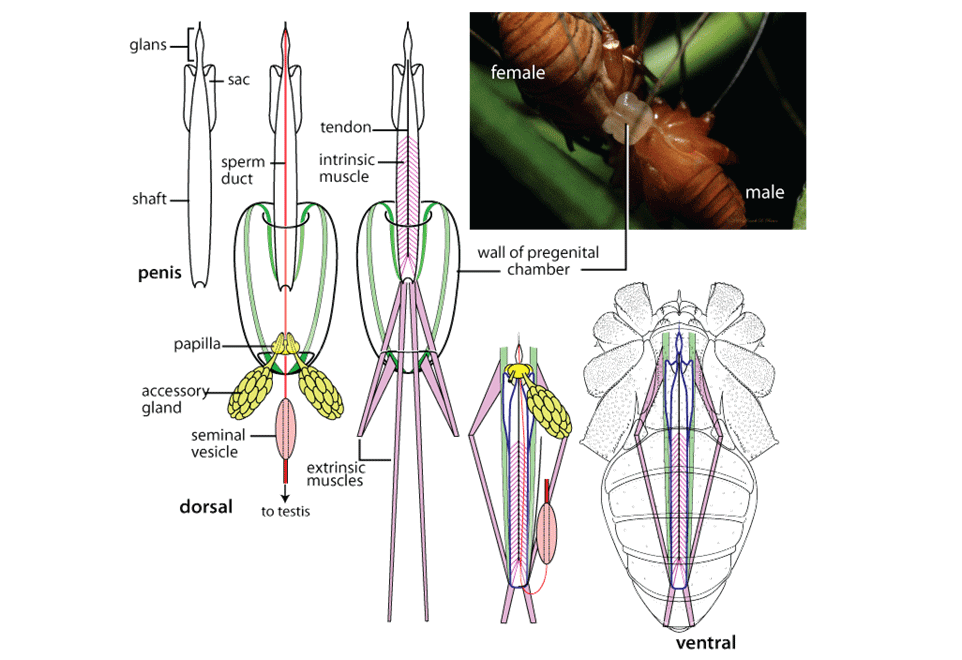
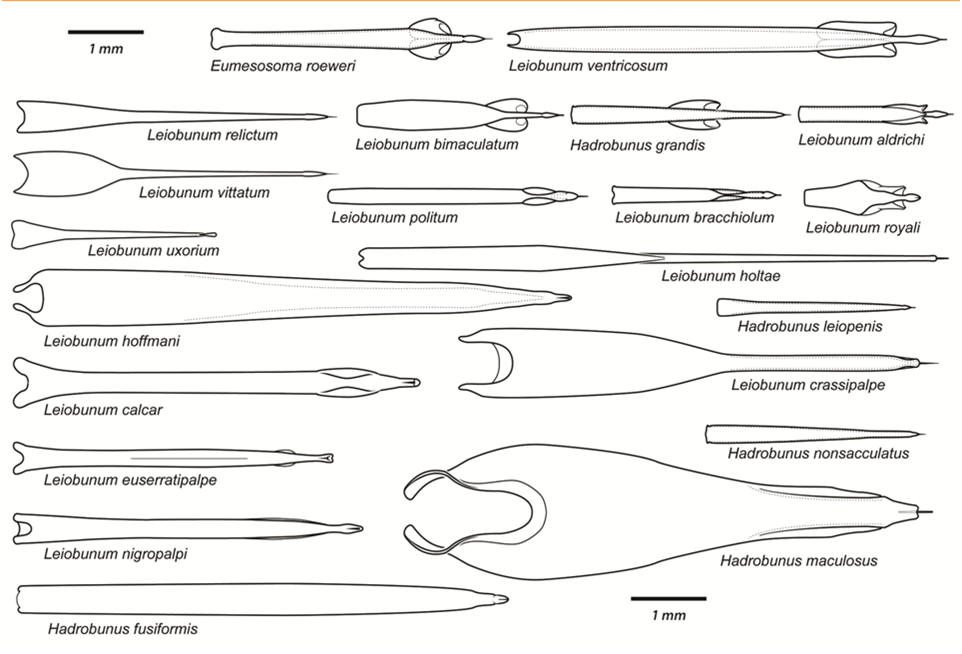
Post by Armando Rosario-Lebron Dr. Jeffrey Shultz, our resident arachnologist, gave us insight into the sex life and morphological diversity of harvestmen or Opiliones, also known as “daddy longlegs”. These arachnids are often ignored and thus provide an open territory for new research. A trek through the woods surrounding the Washington DC metropolitan area will often yield a few harvestmen. According to Dr. Shultz, some of these may be new species that have been overlooked even in the most populated areas of the Eastern United States. Your own back yard could be host to a “new” species of harvestman, undescribed by the scientific community. The Shultz lab now focuses most of their research on the genera Leiobunum and Hadrobunus. Until recently, Hadrobunus was thought to contain only two species, both first described in the 19th century until the recent additions of 11 new species by the Shultz lab. According to Dr. Shultz most species within the genus occur in the southeastern US, with a few lineages reinvading the north following the retreat of the glaciers The pattern suggests that the South served as a glacial refugium. However, what really makes the story interesting is the resulting diversity in the sex lives of both Leiobunum andHadrobunus, which suggests that there has been a hidden “sexual arms race” were males either coerce or entice females to mate with them. In many organisms females tend to “call the shots” in choosing a male that may meet standards for health or increase the chances of offspring and/or egg laying success with nuptial gifts. The healthy plumage of a peacock’s feathers for example may indicate health, “good genes” and a higher success rate for future offspring. This system attempts to ensure that the “best genes” are passed from generation to generation. However, as in all natural systems the rules can be broken and some males have found ways to bypass the filters to mating that females have put up. This drama of romance and coercion is the current focus of the research within the Shultz lab and is apparent within the morphology of harvestmen. Dr. Shultz gives us beautiful illustrations on the genitalia of harvestmen, all critical to the sexual arms race. The diversity within the group is vast – some males have large penises that may even be longer than the body. Other males may have special glands to entice the female to eat and mate. These become tools to penetrate the fortress of the female reproductive system in harvestmen. The females themselves may have bulwarks in the form of an armored operculum or flap covering their pre-genital opening near the mouth. These plates may be notched and sealed shut with immense force by muscles all attaching to an internal structure called an endosternite. As a testament to the strength of the plates, Dr. Shultz noted that during dissection removal of the plate may make a sudden audible pop when pulled open. In some females these may be further modified with the entire structure forming a unit sealed shut and wedged between two sternal plates, effectively locking the structure. Beyond that lies a worm-like ovipositor with a small opening that is the target that the male’s penis has to reach. She uses this ovipositor to both probe the soil and lay her eggs in the right location. The males in response have a penis that extends via hydraulic function. Similar to the diversity of the female defensive mechanisms the male penises vary in the number of muscles they have and the presence of special sacs they use to entice the female to mate. These sacs release a nuptial gift directly into the female's mouth. As she is eating the female will eventually open her operculum and the penis will enter the pregenital chamber. In some species this process of enticing or coercing the female may take hours, in others minutes. Dr. Shultz's research revolves around determining the evolutionary relationships between these harvestmen based on the presence or absence of these sacs. Eastern North America, Central America, Europe and Asia appear to be centers of biodiversity for harvestmen which led to the question: Are the harvestmen of eastern North America a monophyletic group or a phylogenetic mosaic with relationships across the globe? According to Dr. Shultz there is sufficient confidence in recent molecular data to show that most of the North American group is monophyletic or derived from a common ancestor and is not a phylogenetic mosaic. Graduate student Mercedes Burns in the Shultz lab explored a facet of this hypothesis via a phylogenetic analysis of the relationships between harvestmen, focused on the states of the presence or absence of nuptial sacs. In essence two methods were used. One assumed that the “ancestral” state of species is the presence of nuptial sacs, but these could be lost evolutionarily. The second method attempts to determine the rate of change from having sacs to not having sacs and overlays these over the relationships between species of harvestmen on a tree. The results provided evidence that, over time, female barriers were erected in response to males “cheating the system” and no longer using sacs with nuptial gifts. However there was insufficient evidence to suggest that lack of a sac also resulted in males morphologically forcing their way into the female operculum. Dr. Shultz is now collecting data on the strength of male penile protractor muscles and comparing that to female operculum muscles. His laboratory is also working on determining if there is a difference in the flexibility of the harvestmen penis and if this is correlated with an ability to force their way into the operculum. In the end, Dr. Shultz would aims for a “big picture” where he can test whether environmental conditions drive this sexual arms race and create shifts between enticement vs. antagonistic strategies in harvestmen. This may provide insights into sexual selection in other organisms and how changes in the environment can shift reproductive strategy. About Armando
Armando Rosario-Lebron is a Graduate Student within the laboratory of Dr. Cerruti RR Hooks. He is currently studying methods to augment natural enemies of Stink Bugs in agricultural systems. Post by Erik Bergmann In last week’s blog post we looked into how small leaf-tying caterpillars have disproportionate impacts on other arthropod species by modifying their environment. In this week’s colloquium, the new Chair of the Department of Entomology, Dr. Leslie Pick, spoke about her research using another small insect, the humble fruit fly (Drosophilla malanogaster). This fly punches far above its weight class due to its use as a tool to conduct research in the fields of genetics, evolutionary and developmental biology, and even human medical research. Dr. Pick explained that Drosophila essentially is a lab rat, or a model organism. This fruit fly is so highly studied, providing detailed knowledge of the fruit fly’s genetics and development, that it can be used to understand phenomena in research on completely different organisms, like humans. Model organisms are an essential tool for scientists to conduct research that would otherwise be very difficult or impossible with more complex creatures. Dr. Pick and her students were able to identify sections of the fruit fly genome that control insulin and its function regulating sugar levels in their blood. By deleting different combinations of 5 peptides in the fly’s DNA that help with the uptake of sugar, Dr. Pick’s lab found a system that mimics diabetes in humans. They discovered that removal of these peptides didn’t cause instant death of the flies, as it would have in a mammalian species. Instead, they observed a drop in fly body fat, a drop in fertility, delayed development, and an increase in their blood sugar levels. Essentially, the removal of these insulin-like proteins causes the flies to be diabetic and proved that these fruit flies could be a potential model organism for diabetes research! Using this information, Pick’s group genetically modified flies with human genes that help to regulate insulin and sugar levels in human blood when fed a diet with varying levels of sugar. Surprisingly, the flies proved to be incredibly resilient and there was a high degree of conservation in maintaining constant levels of sugar in their blood. This is only the tip of the iceberg on this work with diabetic flies and Dr. Pick’s research. Want to know more about Dr. Pick, her lab, and their research? Click HERE to check it out! Erik Bergmann is a 3rd year MS student studying the feeding patterns of the brown marmorated stink bug, an invasive pest species now found nationwide, on woody ornamental plant species.
Post by Mayda Nathan For shy, cryptic animals that are – at their biggest –an inch long, leaf-tying caterpillars play an outsize role in the tree canopies where they live. Dr. Elisha Sigmon’s recent seminar highlighted the importance of the leaf and silk structures these caterpillars make, both for the caterpillars themselves and for hundreds of other arthropod species that live in forest trees in the Mid-Atlantic region. By fastening together overlapping leaves with silk, caterpillars create a shelter that protects them from predators and harsh climates. Dr. Sigmon’s work has shown that opportunistic insects and spiders quickly find these shelters and move in – even if the original caterpillar is still an occupant. On trees with leaf ties, arthropod abundance and richness can be 3 to 10 times higher than on trees without leaf ties. Just how much leaf ties boost arthropod abundance depends on the host tree species and on the time of year. When she created artificial leaf ties on white oak and beech trees, Dr. Sigmon found that although arthropod diversity within the leaf ties was similar across tree species, arthropod abundance was much higher on white oaks. As the experiment continued into late summer, insects and spiders continued to colonize the leaf ties, boosting arthropod abundance further. Other animals don’t randomly colonize leaf ties, however. After creating artificial leaf ties with and without resident caterpillars, Dr. Sigmon concluded that the composition of arthropods found in the leaf ties depended on the presence of a caterpillar. Specifically, the relative number of herbivores and predators was larger in leaf ties with caterpillars than in those without. What’s more, when she introduced “intruder” caterpillars to leaf ties that already contained a resident, Dr. Sigmon observed caterpillar battles – shoving, hitting, and drumming on the leaf surface. These miniature bouts are a rare documentation of direct interference competition between herbivorous insects, and they point to the leaf ties as an important resource for caterpillars and other arboreal arthropods in Mid-Atlantic forests. Beavers and corals are usually what come to mind when we consider the term “ecosystem engineer”, but the tiny leaf-tying caterpillars may well deserve that label, too. A link to Dr. Sigmon's most recent paper, via BioOne: http://www.bioone.org/doi/abs/10.1603/EN12064 About Mayda:
Mayda Nathan is a PhD student studying the consequences of species range shifts on plant-insect interactions. She currently works with insect pollinators of the black mangrove, which is expanding its range in northern Florida. |
Categories
All
Archives
June 2024
|
Department of Entomology
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290
University of Maryland
4112 Plant Sciences Building
College Park, MD 20742-4454
USA
Telephone: 301.405.3911
Fax: 301.314.9290









 RSS Feed
RSS Feed




